Case Report
Austin J Psychiatry Behav Sci. 2014;1(5): 1024.
EEG and Neurocognitive Changes in Chronic Methamphetamine Abuse
Ceplova Z1, Palenicek T2, Brunovsky M2 and Protopopova D2*
1Department of Psychiatry , Charles University in Prague, Czech Republic
2Prague Psychiatric Center and National Institute of Mental Health, Czech Republic
*Corresponding author: Protopopova D, Department of Arts, Charles University in Prague, Prague Psychiatric Center, Ustavni 91, 18103 Prague, Czech Republic
Received: May 30, 2014; Accepted: June 30, 2014; Published: June 01, 2014
Abstract
Methamphetamine is a potent and highly addictive psychostimulant that has become very popular on the illicit drug market. There is a large amount of literature regarding the neurocognitive changes associated with chronic methamphetamine exposure that are present in currently abstinent individuals. However, the evidence about the possible reversibility of such changes is less clear and only sparse data exists concerning the electroencephalographic (EEG) changes in methamphetamine users. We present a case of a 38 year old male with a 10 year history of continuous abuse of high doses of methamphetamine. The patient has been incapable of adequate social functioning for the last two years and during that period has also been repeatedly admitted to a psychiatric ward due to methamphetamine associated aggressive behavior. After 25 days of abstinence, neurocognitive testing revealed mild cognitive impairment that was most pronounced in psychomotor speed and sequencing in working memory. EEG assessment performed by both visual inspection and quantitative EEG brain mapping showed a slowing of the frequency of dominant occipital activity to the sub alpha frequency band as well as an increase of theta power. No pathological findings were seen on the Magnetic resonance imaging of the brain, however, repeated EEG 20 days later confirmed an abnormal slowing of the dominant alpha frequency. Our findings are in accordance with the limited data available in the literature. However, further studies are needed to confirm the utility of EEG as a marker of methamphetamine toxicity, as well as the association between EEG findings and the neurocognitive performance and the reversibility of both variables in the case of sustained abstinence.
Keywords: Methamphetamine; Cognition; Electroencephalography
Case Presentation
A 38 year old male patient with a previous psychiatric history of drug abuse classified as methamphetamine and multiple drug dependence syndrome (F15.2 and F19.2 according to 10th revision of the International Statistical Classification of Diseases and Related Health Problems [ICD-10]) was admitted to the psychiatric hospital in Czech Republic in January 2014. The reason for the initial admission was a repeated expression of verbal aggressive behavior toward his family members and destruction of property. Similar reasons had led to four previous psychiatric hospitalizations at the same facility in 2013. In all four previous occasions, the patient was diagnosed with methamphetamine intoxication and methamphetamine dependence syndrome (F15.0 and F15.2 according to ICD-10) and was discharged after detoxification on refusing any further therapeutic intervention.
At the time of the current admission the patient was still a frequent methamphetamine user with a ten year history of continuous abuse (sniffing up to 1 gram daily). He also claimed a two year lasting episode of excessive alcohol abuse (up to half a liter of spirit daily) which ended three years ago. The patient had no family history of psychiatric disorders and a negative personal history of any medical conditions other than minor injuries during car crashes. He graduated with a degree in economics at a private university with average results and subsequently had a prospering career in real estate until the year 2012. Since then, possibly in association with pronounced family conflicts, he increased his drug use, lost most of his social contacts, interest in his job and as a consequence he was declared bankrupt.
After admission to the psychiatric hospital, the patient underwent basic physical and neurological examination together with blood and urine analysis that included the standard serum biochemistry, complete blood count with differential, C-reactive protein, basic urinalysis, viral hepatitis screening and screening for syphilis. There were no pathological findings except from slightly elevated bilirubin found by blood analysis. The urine level of amphetamines was 1534 ug/l, benzodiazepines 413 mg/l and the urine screening was negative for opiates and cannabinoids. The clinical symptoms on admission included acceleration of speech and motor activity, irritability with occasional hostile attitude, exaggerated self-opinion, flight of ideas, poor attention and insomnia. No psychotic symptoms were present and the admission diagnoses were F15.0, F15.2 and F19.2. However, after 14 days of detoxification that consisted of close monitoring of vital signs and low doses of benzodiazepines and risperidone, the patient remained accelerated, irritable with feelings of superiority and with the tendency to organize the patients and the staff on the ward. Therefore, the valproate was initiated and the patient was transferred to a superior psychiatric clinical facility to exclude comorbid bipolar affective disorder (F31.3 according to ICD-10). At the time of transfer, he was medicated with risperidone 4 mg and valproate 750 mg daily. The valproate was further titrated to achieve the therapeutic dose according to blood levels with 289.9μmol/l at a dose of 2000 mg/day and 630μmol/l at a dose of 3000 mg/day (therapeutic range: 346.5 - 693μmol/l). The risperidone dose was initially increased to 5 mg daily to control the manic symptoms and slowly discontinued afterwards. The patient's psychopathology started to improve gradually after 4 weeks from the initial admission to psychiatric hospital (approximately two weeks after valproate initiation) and three weeks later he was discharged stable, euthymic and on 3000 mg of valproate daily.
Regarding the examinations, formal neuropsychological testing and EEG were arranged for the patient as a part of standard diagnostic process. The neuropsychological tests were performed a week after patient`s admission to psychiatric clinic when he was clear from methamphetamine for 25 days. The battery of tests included the Wechsler-Adult Intelligence Scale-III (WAIS-III) - general intelligence test, Trail Making Test (TMT) - test of visual conceptual and visual motor tracking, The Rey Complex Figure Test (RCFT) - test of visual perception and memory, executive functions and visual motor and constructional skills, Verbal fluency test - test of executive functioning and language ability test. The results of TMT (t(A)= 40s, t(B)= 163s), RCFT (RS 29,5-8,5-7,5 PS: 7-5-5), and Verbal fluency test (RS 9-5-6 Σ20 repetions:0, confabulations:0) were below average. In the WAIS-III test the patient's contemporary intellectual performance was within the low average and average range (Full Scale IQ: 85). The lowest performance was seen in processing speed, the highest in perceptual organization. Verbal (VIQ: 88) and Performance IQ (PIQ: 84) were equable. Considerable intersubtest variability indicated the unreliability of cognitive functions. Summarized, current cognitive performance fell within the low average and average range. In regard to his achieved education and former employment, it could be assumed that the performance did not match the premorbid level of the patient's cognitive abilities. We found impairment in executive functions, working memory and psychomotor speed.
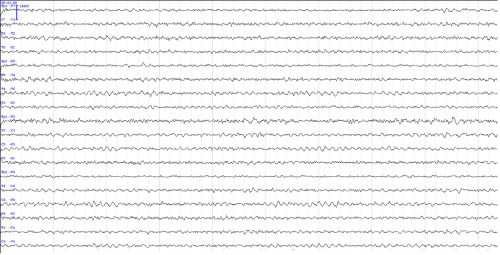
Due to cognitive decline found by psychological testing, a magnetic resonance imaging (MRI) structural scan of the brain was performed to exclude any underlying organic condition. The MRI showed no pathological findings. On the contrary, visual inspection of electroencephalographic (EEG*) recording done 26 days after patient`s methamphetamine discontinuation yielded abnormal EEG result with slowing of the dominant occipital activity and diffuse increase of slow-wave activity (Figure 1). During maximal wakefulness the dominant occipital background activity consisted of a 7.8-8.3 Hz sub alpha rhythm (as shown by spectral analysis). The EEG signal was symmetrically but only slightly attenuated by the changing condition from eyes open to eyes closed and vice versa. Although a high level of drowsiness was observed during this recording the slowing of dominant activity was clearly evident. Quantitative EEG brain mapping of the first 5 minutes of recording showed an increase of theta-slow alpha power (7.32 - 8.79 Hz) in the parietooccipital region and increase of theta power (5.37-6.35 Hz) in frontocentral region (Figure 2a). It is important to note that the EEG was performed while the patient was on a stable daily medication of 5 mg of risperidone and 2000 mg of valproate. The EEG was repeated 20 days later with comparable but slightly improved results. The dominant frequency was variable but still partly in the sub alpha frequency band (7.3 - 8.8 Hz). Brain mapping of the first 5 min of recording yielded similar power values with mild increase in 8.79 - 9.28 Hz frequency range (Figure 2b). At the time of the second recording the patient was on a daily dose of only 3000 mg of valproate.
Figure 1: Example of a typical patient's electroencephalogram during wakefulness 26 days after methamphetamine discontinuation (longitudinal montage, 10s of EEG).
Figure 2: Brain mapping performed in the first 5 min of EEG during wakefulness A) 26 days and B) 46 days after methamphetamine discontinuation. Images show mapping of absolute power values in frequency range of 0.49 - 14.16 Hz (with ~0.49 Hz resolution) the scale on the right side shows the absolute power values ranging from 0 to 10.0 μV/Hz. The red color indicates the maximum of the power, and the blue color the minimum (Wave Finder v.2.33, brain mapping was calculated against the average reference electrode).
The final diagnosis of the patient was changed to a residual affective disorder related to the abuse of amphetamines (F15.7 according to ICD-10). That diagnosis was based on patient`s psychiatric history (manic symptomatology or aggression exclusively in association with methamphetamine use, no depressive symptoms in the history), exclusion of organic etiology by magnetic resonance imaging (MRI) and findings corresponding with chronic methamphetamine use revealed by EEG and psychological testing. We couldn`t exclude the possibility that the patient underwent the first manic episode of bipolar disorder that was only triggered by methamphetamine use, however he didn`t fulfill the diagnostic criteria for bipolar disorder at the time of his hospitalization. It would require a longer period of abstinence to confirm an affective disorder per se.
The patient signed informed consent and agreed freely to all performed examinations. Institutional Review Board approval was not required as there was no deviation from a standard care and published data didn`t allow an identification of a patient.
* The EEG (eyes closed condition) was recorded using the standard 19 electrodes of a 10/20 system with a "BioSDA09" 36 channel amplifier (M&I spol. s.r.o., Prague, Czech Republic), and with primary sampling at 25 kHz secondarily down sampled to 1 kHz for further viewing and processing. Visual inspection of EEG and subsequent quantitative analyses were performed in Wave Finder v.2.33 software (Unimedis, Prague). Brain mapping was performed after digital filtering 0.5-30 Hz against the average reference electrode
Discussion
The observed slowing of EEG along with the cognitive deficit leads us to a hypothesis of an organic etiology to the disorder. However, since no changes on MRI were observed, we believe these findings could be related to the methamphetamine toxicity. Except for physical effects such as anorexia, sweating or an irregular heartbeat, methamphetamine is typically associated with psychological effects including dysphoria, restlessness, insomnia, grandiosity, anxiety, psychosis, and violent behavior. Moreover, unlike amphetamine, methamphetamine is directly neurotoxic to dopamine neurons, causing dopamine transporter reduction. There is growing evidence that such brain changes could interfere with the cognitive functioning of the affected individuals [1,2]. Scott et al. [3] found in their metaanalysis that methamphetamine users scored significantly lower in cognitive tests compared to the control group. The effects were largest in learning abilities, executive functions, memory and processing speed. The evidence for cognitive improvement associated with abstinence from methamphetamine is mixed [4]. We observed similar cognitive changes to those described in the literature in a methamphetamine-dependent patient after almost a month of abstinence.
While there is a vast amount of literature on the psychopathology and neurocognitive changes of current and former amphetamine/ methamphetamine users, little evidence is available on the long-term EEG changes in these subjects. The only published data about EEG changes in abstinent methamphetamine users described an increase in theta and delta power after 4 days of abstinence. Also, 64% of all users compared to 18% of non-users had abnormal EEG [5]. These findings are in a good accordance with what we have observed. However, our main finding in the case of our patient was that these changes persisted for about a month after discontinuation of methamphetamine and were associated with a significant cognitive decline.
It is of note that during the first EEG examination the patient was on 5 mg of risperidone daily. Risperidone itself increases the power in the theta and delta bands [6,7]. However, the second evaluation of EEG performed without the drug showed a similar profile to the first one. Another theoretical confounding factor might be valproate. On the contrary to risperidone, valproate is not typically associated with EEG slowing unless in cases of acute toxicity [8-10]. However, this patient had normal serum levels of valproate and did not show any other signs of valproate toxicity, therefore the influence of the drug on EEG is unlikely. Theoretically, his anterior excessive alcohol use may have also contributed to some CNS toxicity. It has been repeatedly shown that EEG of alcoholics typically shows increased theta power and a deceleration of alpha [11]. However, at the time of examination, alcohol had not been used in excessive amounts for more than 3 years and no cortical atrophy was observed, therefore we believe alcohol could have only a minor effect.
To conclude, we report a remarkable cognitive decline in association with a slowing of EEG dominant frequency, which persisted for 46 days after methamphetamine discontinuation. This indicates that long-term use of high doses of methamphetamine can induce severe persisting neurotoxicity.
Acknowledgment
This work was supported by projects IGA MHCR NT/13897, MICR VG20122015080 and VG20122015075, MH CZ - DRO (PCP, 00023752) and PRVOUK P34. We thank Craig Hampson BSc (Hons) for his helpful comments and language correction. No authors have any involvement, financial or otherwise, that might constitute a conflict of interest in the work contained in this manuscript.
References
- King G, Alicata D, Cloak C, Chang L. Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology (Berl). 2010; 212: 243-249.
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling Wm, et al. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000; 9: 222-231.
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007; 17: 275-297.
- Dean AC, Groman SM, Morales AM, London ED. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013; 38: 259-274.
- Newton TF, Cook IA, Kalechstein AD, Duran S, Monroy F, Ling W, et al. Quantitative EEG abnormalities in recently abstinent methamphetamine dependent individuals. Clin Neurophysiol. 2003; 114: 410-415.
- Hughes AM, Lynch P, Rhodes J, Ervine CM, Yates RA. Electroencephalographic and psychomotor effects of chlorpromazine and risperidone relative to placebo in normal healthy volunteers. Br J Clin Pharmacol. 1999; 48: 323-330.
- Czobor P, Volavka J. Quantitative electroencephalogram examination of effects of risperidone in schizophrenic patients. J Clin Psychopharmacol. 1993; 13: 332-342.
- Wu X, Ma JJ. Sodium valproate: quantitative EEG and serum levels in volunteers and epileptics. Clin Electroencephalogr. 1993; 24: 93-99.
- Sannita WG, Gervasio L, Zagnoni P. Quantitative EEG effects and plasma concentration of sodium valproate: acute and long-term administration to epileptic patients. Neuropsychobiology. 1989; 22: 231-235.
- Adams DJ, Luders H, Pippenger C. Sodium valproate in the treatment of intractable seizure disorders: a clinical and electroencephalographic study. Neurology. 1978; 28: 152-157.
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H, et al. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005; 116: 993-1018.