
Research Article
Austin J Psychiatry Behav Sci. 2016; 3(1): 1048.
Cost-Effectiveness of Treatment Options for ADHD: A Systematic Literature Review
Klora M¹*, Zeidler J¹ and Greiner W²
¹Center for Health Economics Research Hannover (CHERH), Leibniz University Hannover, Germany
²Faculty of Health Sciences, University of Bielefeld, Germany
*Corresponding author: Mike Klora, Center for Health Economics Research Hannover (CHERH), Leibniz University Hannover, Otto-Brenner-Str. 1, Hannover, 30159, Germany
Received: January 12, 2016; Accepted: March 09, 2016; Published: March 11, 2016
Abstract
Backround: Interest in cost-effective Attention Deficit Hyperactivity Disorder (ADHD) treatment is rising, and the reduction of costs is important due to increasing health care expenditures. Treatment strategies for ADHD patients consist of medication, behavioral treatment, and combined treatment and have to be compared regarding cost-effectiveness to enable reliable decisions on appropriate treatment.
Objective: The objective is to present evidence on cost-effectiveness of ADHD treatment strategies and to discuss the potential for economic optimization from an international perspective.
Methods: A literature review was conducted within the German Institute of Medical Documentation and Information (DIMDI) literature database and presented according to the PRISMA scheme. Inclusion criteria consist of costeffectiveness and the potential for cost reduction by treatment.
Results: While there is evidence for the cost-effectiveness of pharmacotherapies, there is less information regarding the cost-effectiveness of other treatment options, such as behavioral treatment. Medication is cost-effective according to the international threshold values. Atomoxetine, dextroamphetamine and guanfacine represent an alternative or supplement treatment to methylphenidate depending on patient characteristics.
Discussion: There is only small evidence for the cost-effectiveness of multimodal treatment for ADHD patients worldwide. Cost factors such as comorbidities, hospitalization and compliance have to be considered in addition for the choice of therapy.
Conclusion: The cost-effectiveness of ADHD medications is well documented, whereas studies on the behavioral measures and multimodal therapy are lacking. There is an urgent need for evaluation, especially, because the multimodal therapy is defined as an important element treatment by experts and guidelines.
Keywords: Attention Deficit Hyperactivity Disorder (ADHD); Atomoxetine; Behavioral treatment; Cost-effectiveness; Methylphenidate
Abbreviations
ADHD: Attention Deficit Hyperactivity Disorder; DALY: Disability-Adjusted Life Years; DIMDI: German Institute of Medical Documentation and Information; DSM: Diagnostic and Statistical Manual of Mental Disorders; GIR: Guanfacine Immediate-Release; GRX: Guanfacine Extended-Release; ICD: International Classification of Diseases; ICER: Incremental Cost-Effectiveness Ratio; MTA: Multimodal Treatment Study of Children with ADHD; MEPS: Medical Expenditure Panel Survey; N: Number; OROS: Long-Acting Osmotic Release Oral System; PICOS: Participants-Interventions- Comparisons-Outcomes-Study; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QALY: Quality-Adjusted Life Year
Introduction
Relevance of ADHD
Attention Deficit Hyperactivity Disorder (ADHD) is one of the most commonly diagnosed mental disorders in children and adolescents. The prevalence is approximately 3 to 5% worldwide [1,2]. The rate of persistence into adulthood varies from 30 to 80% across countries, illustrating that ADHD persists in the elderly population [3,4]. A bibliometric study demonstrates the increase in international publications and productivity in the field of ADHD research from 1980 to 2005 [5]. However, there has been no evidence to suggest an increase in the number of children with ADHD, although such hypotheses have been made due to the rising prescriptions of ADHD medications (e.g. methylphenidate) [6]. Despite the majority of the ADHD disease burden occurring in childhood, its overall prevalence warrants the attention of decision makers regarding early intervention, treatment [7], cost reduction potential, and increased therapeutic efficacy.
The symptoms of ADHD are mainly characterized by a deficit in attention, lack of persistence in activities, impulsivity, and hyperactivity. Attention deficit hyperactivity disorder is found as code F90.x in the International Classification of Diseases, 10th Revision (ICD-10), and 314.x in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) [8,9]. The diagnosis is more common in males [10], and medication, behavioral treatment, and combined therapy are the main treatment options. Typical first and second line medications are methylphenidate and atomoxetine in most parts of the world. The annual treatment costs associated with atomoxetine are higher than those for methylphenidate [7,11–13]. Other treatment strategies are conceivable, for example in countries where the restriction of resources renders cheaper treatments to be viable alternatives. Furthermore, comorbidities influence treatment strategy, thereby contributing to a higher severity of illness and higher treatment costs [14]. Typical comorbidities include social behavioral disorders, oppositional disorder, mood disorders (i.e., bipolar disorder and depression), anxiety disorders, tic disorders, learning disabilities, and enuresis. The proportion of comorbidities differs by age [15,16].
In 2000, the costs associated with ADHD in the USA were 30.1 billion € and encompassed treatment costs, other healthcare costs, and work loss costs [17]. A more recent systematic literature review provides increased overall national annual incremental costs associated with ADHD, which ranged from 108 to 201 billion € in 2010 [18]. Total direct costs in Germany for the age group below 15 years were 287 million € in 2006. This amount includes costs associated with inpatient treatment, outpatient treatment, medication, and other treatment costs [19]. However, economic burden estimates differ depending on which costs are included and which age groups are considered, as well as from which country this information is recruited.
There are many studies focusing on the economic burden of ADHD, and several reviews can be identified [20,21]. Cost projection studies by Schlander et al., (2007) pointed out rising medication costs, which have been the main impetus for considering the costeffectiveness of medication strategies [22–25]. In summary, existing reviews provide information regarding total costs associated with ADHD or compare the cost-effectiveness of medication strategies but only little evidence for potential reduction in treatment costs of all other forms of treatment (including behavioral treatment)is published until now. Additionally, there are methodological reviews regarding aspects of “assessing the efficacy of treatments for ADHD” as well as reviews regarding the “international guidelines on ADHD” [26,27]. The literature has provided information regarding the mean and total cost associated with ADHD first- and second-line treatments by country. However, limitations in these medication studies exist. For example, only short-term effects were considered and no evidence on the long-term effects e.g. compared to behavioral options, which may be more effective in the long-term, is published. Furthermore, most studies consist of indirect treatment comparisons, such as no treatment and no medications.
Therefore, the objective and rationale of this review is to provide a systematic overview of the potential strategies to reduce treatment costs for ADHD and their cost-effectiveness as well as to provide a speculative view on this topic for cost reduction, if monetary values are missing.
The following questions were addressed according to the Participants-Interventions-Comparisons-Outcomes-Study (PICOS) design scheme. The participants were international ADHD patients. Our main questions were, which therapy is the most cost-effective and how is cost reduction potential affected by ADHD characteristics and behavior (e.g., comorbidities, and persistent or non-persistent patients). The considered interventions were medication strategies, behavioral therapy, and innovative treatments, such as diet or sports. Our main questions regarding comparisons were which the most cost-effective therapy is and for which treatment strategies do studies about cost-effectiveness exist? For outcomes we observed cost-effectiveness across treatments, while the study design consisted of surveys and economic modelling. The different methods of each study design had to be recognized as well.
Materials and Methods
We conducted a literature review in German and English within the German Institute of Medical Documentation and Information (DIMDI) literature database, which is a commonly used and comprehensive database with international focus (search date: 08.01.2015). No time limit was imposed on the search period. The DIMDI literature database consists of MEDLINE, BIOSIS Previews, EMBASE Alert, EMBASE, GMS, GMS Meetings, and Sci Search. Study characteristics were cost, disease, and treatment focused. The English and German combined literature search terms were [? Cost? or ?Kost? or Ressourcen? or resource? or Krankheitskosten?] [ADHD or ADHS or Attention deficit hyperactivity disorder or Aufmerksamkeitsdefizit-/Hyperaktivitatss?] [Treatment? or Behandlung?]. During the initial inspection, we searched for duplicates. Afterwards, the title of every identified publication was scanned for relevance regarding cost-effectiveness and the potential for cost reduction by treatment before scanning for the same aspects in the abstracts. This was done because only evidence-based monetary value can be used in clinical practice to reduce treatment costs. Without a monetary value, only a speculative view remains as to whether there was any reduction in treatment cost potential. If the publication was available, papers included by the relevance of the abstract were scanned for relevance regarding the content of the publication as a whole. The paper was included in the study sample (Table 1) if clear evidence was shown by an issued monetary value for treatment cost reduction compared to the standard treatment or no treatment. Only original studies were included. The rationale for this was that the contents of posters or abstracts do not include full analyses or may consist of preliminary results. Reviews were excluded because the original paper had already been covered by our literature review or were added manually. Currencies that were not denominated in Euros were converted at the exchange rate of the evaluation year. The methods of this literature review are defined according to the PRISMA scheme.
Results
Evidence of Cost-Effectiveness in ADHD Treatment
The database search revealed 582 studies (1063 studies before the duplicates were excluded). After manual search and afterwards scanning for relevance of title and abstract, 151 studies remained.19studies were not available as full publication, and 115 were excluded (see the Flow Chart for exclusion reasons). Finally, 17 publications were identified to show clear evidence for reduction of treatment costs (Table 1 and 2). These studies consisted predominantly of surveys and health economic modelling. The primary reason for exclusion was a lack of focus on costs and reduction of treatment costs compared to standard treatment (e.g., no information on costeffectiveness) (Figure 1). However, these studies were considered in the discussion, for example, if a new efficient treatment approach was investigated and a reduction in treatment costs could be assumed.
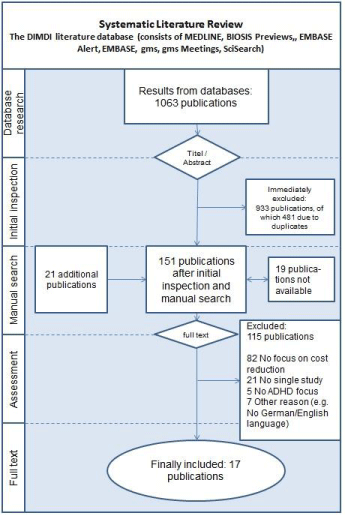
Figure 1: Flow chart.
The 17 identified studies varied in their characteristics regarding treatment option comparison, geographical location, study type, perspective and patients characteristics (age and type of the ADHD disease) (Table 1 and 2).
Study
Sample Size
Age
Time period
Perspective
Country
Data source
Chan et al. 2002
5,439 children
(165 children with ADHD)5 to 20
1996
payers and patients
United States
Medical Expenditure Panel
SurveyVanoverbeke et al. 2003
8 UK psychiatrists and
paediatricians
6 to 16
2001
payer
Great Britain
Delphi Panel
Donnelly et al. 2004
4500 children and adolescents
(Original survey)
4 to 17
2000
payers and patients
Australia
National Survey of
Mental Health and Wellbeing – Child and Adolescent Component
Narayan et al. 2004
1
9
2003
social
United States
Literature review for data inputs
Jensen et al. 2005
579
7 to 9.9
2002
social/payer
United States
NIMH’s Multimodal Treatment
Study of Children With ADHD (MTA Study)*Stevens et al. 2005
221 children (short-acting)
153 children (longer-acting)3 to 18
2000-2001
social
United States
Medical Expenditure Panel
SurveyWu et al. 2007
4,569 patients
18 to 64
2004
payers
United States
Claims data maintained
by Ingenix, Inc.Cotrell et al. 2008
Prasad et al. 2009
83 parents
not specified
not specified
social
England and Wales
National Health Service
Faber et al. 2008
83 parents
8+
10 years
social
Netherlands
Expert panel consisting of three pediatricians and
two child psychiatristsHong et al. 2009
83 parents
not specified
not specified
social
Spain
National Health Service
Myrén et al. 2010
99 pediatric ADHD patients
(questionnaire filled out by parents)2004
social
Sweden
Swedish County Council’s official
statistics and databasesSikirica et al. 2012 (a)
22,622
6 to 12
2005-2009
payers
United States
US
commercial medical/pharmacy claims databaseSikirica et al. 2012 (b)
461
not specified
not specified
payer
United States
US
third-party payerSikirica et al. 2013
3,087
6 to 17
Nov 2009 - Dec 2010
payer
United States
Truven
Health MarketScan databaseBraun et al. 2013
3,407
newly diagnosed patients6 to 17
2007
payer
Germany
Second largest German sickness fund
Sikirica et al. 2014
849
13 to 17
2005-2009
payer
United States
Truven Health Markets can Commercial Claims and Encounters database
Table 1: Study overview.
Study
Studied treatment
Approach
Relevant year
Costs
Adjusted costs
Findings
Chan et al. 2002
Stimulants vs.
no stimulants
Health care expenditures derived from panel data
(inpatient costs, outpatient costs,drug costs and out of-pocket payments)
1996
Children with ADHD treated with stimulants: 1,113 €
Children with ADHD with no stimulants: 412 €
(First converted from $ to DM for the year 1996 and then to € by the first exchange rate of 1€ =1,95583 DM, because introduction of the euro was first on January, 1999)Not for this comparison
ADHD patients with no stimulant treatment show lower health care expenditures
and significant lower resource use regarding outpatient visits and prescriptionsVanoverbeke et al. 2003
Methylphenidate and Behavioral treatment
Incidence based
medical decision tree model
2001
Starting with MPH-OROS and switch to:
behavioral treatment: 2,662 €total costs
combination treatment: 4,122 € total costs
other drug: 2,209 € total costs
No adjustment for patient’s characteristics
A switch to behavioral treatment should be avoided.
Donnelly et al. 2004
Dexamphetamine
(DEX) and Methylphenidate (MPH) interventions
compared to current practice
Meta-analysis of
randomized controlled trials and
Incremental Cost Effectiveness
Ratio (ICER)
2000
The ICER for DEX is 2,579 €/DALY saved) and for
MPH is 9,437 €/DALY saved
Uncertainty parameter included
MPH and DEX are cost-effective interventions for childhood ADHD. DEX is
more cost-effective than MPH, although if MPH were listed at a lower price on the
Pharmaceutical Benefits Scheme it would become more cost-effective. DEX is more costly than MPH for
the government, but much less costly for the patient.
Narayan et al. 2004
Treatment with amphetamine/dextroamphetamine compared to no treatment
Decision-tree analysis with ICER per QALY
2003
AMP/DEX versus no treatment (as MPH IR is dominated):
19,433 €
No adjustment for patient’s characteristics
Treatment with amphetamine/dextroamphetamine or methylphenidate is cost effective versus no treatment.
Methylphenidate is dominated by amphetamine/dextroamphetamine therypy in the base case.
Jensen et al. 2005
Major forms of ADHD treatments
(Medical Management, Intensive Behavioral Treatment, Combined Medical
Management, Behavioral Treatment, Routine Community Care)The societal perspective was used to determine the costs for this study derived by the NIMH’s Multimodal Treatment
Study of Children with ADHD (MTA Study)2002
Cost per child per treatment arm for children with ADHD during 14 months of treatment :
- Medical Management: 1,280 €
- Intensive Behavioral
Treatment: 7,577 €
- Combined Medical
Management and
Behavioral Treatment: 8,487 €
- Routine Community Care: 1,161 €No adjustment for patient’s characteristics
Medical management treatment,
although not as effective as combined
medical management and behavioral
treatment, is likely to be more cost-effective
in routine treatment for children
with ADHD, particularly those without comorbid
disorders. For some children with
comorbid disorders, it may be cost-effective
to provide combination treatmentStevens et al. 2005
Short-acting stimulants vs. longer-acting stimulants
Adjusted healthcare expenditures (inpatient, outpatient, drug costs and out-of-pocket payments ) derived from panel data
2000-2001
mean total expenditures:
short-acting-stimulants user: 1,746 €
longer-acting-stimulants user: 2,271 €Adjusted for patient’s characteristics (income, health status and ethnicity)
Longer-acting stimulants user show higher total healthcare expenditures
Wu et al. 2007
Extended-release methylphenidate
(osmotic release oral system-MPH), mixed amphetamine salts extended
release (MAS-XR), or atomoxetineGeneralized linear models (GLMs) to compare costs of adults receiving alternative therapies (medical costs and drug costs (6 month time period) derived from claims database of 5 million beneficiaries.
2004
Risk-adjusted mean medical costs, excluding drug costs, for adults
treated with OROS-MPH were 114 € less (10.4%, 982 € vs. 1,096 €) compared
with MAS-XR (P = 0.022) and 106 € less (9.8%, 982 € vs. 1,088 €) compared
with atomoxetine (P = 0.033); risk-adjusted mean medical costs were not
significantly different between MAS-XR and atomoxetine.Adjusted for
demographic characteristics, substance abuse, depression, and the Charlson
Comorbidity Index
Adults treated with
OROS-MPH had, on average, slightly lower medical and total medical and
drug costs than those treated with MAS-XR or atomoxetine over the 6-month
period after drug therapy initiation.Cotrell et al. 2008
Prasad et al. 2009
Atomoxetine vs. Methylphenidate
(immediate-release; extended release)Incremental cost per QALY using Monte Carlo
simulation and Markov model2004
atomoxetine algorithm compared with
stimulant exposed population:
- immediate-release Methylphenidate (MPH):22,924 €
- with extended release MPH: 19,938 €- atomoxetine compared to defaximine (i.e. failed on either IR-MPH or XR-MPH): 22,025 €
- atomoxetine compared to no treatment: 16,982 € to 18,230 €
-
Atomoxetine is an effective alternative across a range of ADHD patients
and offers value-for-moneyFaber et al. 2008
Long-acting
methylphenidate osmotic release oral system for youths
with ADHD for whom treatment with immediate-release (IR) methylphenidate is suboptimalMarkov model to obtain ICER per QALY
2005
The ICER of methylphenidate-OROS treatment in youths with ADHD
for whom treatment with IR methylphenidate is suboptimal was 2,004 € per
QALY. Total costs after 10 years were 15,739 € for the IR methylphenidate
pathway and 16,015 € for the methylphenidate-OROS pathway.-
Methylphenidate-OROS is a cost-effective treatment for youths with
ADHD for whom treatment with IR methylphenidate is suboptimal. Higher
medication costs of methylphenidate-OROS were compensated by savings on
resource use, yielding similar 10-year costs compared with treatment with IR
methylphenidate.Hong et al. 2009
Atomoxetine vs. Methylphenidate (immediate-release; extended release),
no medicationIncremental cost per QALY using Monte Carlo
simulation and Markov model2004
incremental cost per QALY gained for the atomoxetine algorithm (here only stimulant-naive patients are provided) compared with:
- immediate-release Methylphenidate (MPH): 34,308 €
- with extended release MPH: 24,310 €
atomoxetine compared to no treatment: 23,323 € to 23,820 €-
Atomoxetine is an effective alternative across a range of ADHD patients
and offers value-for-moneyMyrén et al. 2010
Atomoxetine
Resource utilization was derived by questionnaire
Published unit costs/prices were used to calculate costs
2004
On treatment with atomoxetine (no medication costs):
828 € at baseline, 495 € at 10 weeks, 266 € at 25 weeks and 224 € at 49 weeksNo adjustment for patient’s characteristics
Atomoxetine together with parental psychoeducation reduces nonmedication costs associated with ADHD
Sikirica et al. 2012 (a)
Atypical Antipsychotics (AAP) vs. Non-Antipsychotics
Annual resource utilization was compared using McNamara’s test and Poisson regression
2010
The AAP group
incurred higher all-cause mean medical, prescription drug, and total health
care costs compared with the non-antipsychotic group (2,332 € vs. 1,689 €;
2,901 € vs. 1,893 €; 5,233 vs. 3,538, respectively; all P < 0.001)No adjustment for patient’s characteristics
Stimulant-treated children with ADHD who switched to or
augmented with AAPs versus non-antipsychotics had significantly greater
rates of subsequent augmentation and health care resource utilization as
well as higher total health care costsSikirica et al. 2012 (b)
Guanfacine Extended
Release as an Adjunctive Therapy to a
Stimulant Compared with Stimulant
MonotherapyICER per QALY within a 1-year Markov model
2010
Adding
GXR to existing stimulant monotherapy was associated with an incremental
drug cost of 767 € but a lower medical cost of 94 €, resulting in a total
incremental cost of 673 € at 1 year-
The adjunctive therapy of GXR with
stimulants is a cost-effective treatment based on a willingness-to-pay threshold
of 37,732 €/QALY. This may address an unmet need among patients
with suboptimal response to stimulant monotherapySikirica et al. 2013
Guanfacine Immediate Release (GIR) versus
Guanfacine Extended Release (GXR)Claims data analyses (medical service and prescription
drug costs)2010
GIR users
incurred significantly lower all-cause pharmacy costs (P <.001)
but significantly higher medical costs (P = .009), resulting in no
significant difference in total all-cause healthcare costs (P = .068)
between the 2 groups (GIR 3,180 € vs GRX 2,969 €)Unadjusted results shown here
Total all-cause healthcare costs were comparable
between the 2 groupsBraun et al. 2013
Drug treatment–persistent, drug treatment–
no persistent, and nondrug treatmentThe differences
in costs and resource utilization are reported in a descriptive manner, with paired and unpaired
2-sample Wilcoxon tests used. Claims data analyses were conducted.2007
Significant average savings of 187 €/year in overall
costs (P = 0.05) were noted for the drug treatment–persistent group compared with the drug treatment–no persistent group. These mean savings were
€739/year and €552/year (drug treatment–persistent group and drug treatment–no persistent group, respectively)
compared with nondrug-treated patients.No adjustment for patient’s characteristics
There are potential cost-savings benefits
when patients are treatment persistentSikirica et al. 2014
patients who switched to or augmented with atypical antipsychotics compared to those who switched to or augmented with no antipsychotic medication
Retrospective cohort study
2010
The AAP cohort incurred significantly higher mean annual medical (2,733 € vs 2,499 €), drug (3,256 € vs 2,176 € and total healthcare (5,990 € vs 4,676 €) costs.
Propensity-Score-Matching
Stimulant-treated adolescents with ADHD who switched to or augmented with AAPs had significantly costs compared with the non-antipsychotic cohort
Table 2: Costs for studied treatment option.
The focus of most of the studies was on the cost-effectiveness of different medications or mean costs. The cost-effectiveness of different agents were compared among each other or versus no treatment as well as among different formulations in the same class (i.e., whether the agent was short- or long-acting (Table 2)).
Medication
One study concluded that the total annual treatment costs associated with pharma co-stimulant users are higher than the costs associated with patients not using stimulant medication. The found difference was significant and took value of 701 €. The information on costs was retrieved from the Medical Expenditure Panel Survey (MEPS) which contains representative data on costs for the US population including out-of-pocket payments [28]. There is significant higher resource use by patients with stimulant treatment. Therefore, a higher severity of disease can be assumed.
Comparision of atomoxetine, methylphenidate and dexampthetamine
Furthermore, within the United Kingdom, atomoxetine is a costeffective treatment option compared to methylphenidate, although atomoxetine is less effective in first-line patients. Atomoxetine is a treatment option with a greater time of therapeutic response. Markov models were used for estimation of the costs and benefits of atomoxetine including 18 different health states [29,30]. These results were confirmed by Spanish data using the same methods [31]. However, atomoxetine treatment is associated with higher treatment costs as well Claims data analyses (5 million beneficiaries from 31 large self-insured employers) from the USA show that for the year 2004, adults treated with extended-release methylphenidate had lower risk-adjusted total costs in a 6-month period (i.e. 982 € vs. 1,088 € for patients treated with atomoxetine). Even when compared to patients treated with mixed amphetamine salts, the costs associated with patients treated with extended-release methylphenidate were 114 € less. [32]. More over cost-effectiveness is higher for dexamphetamine than for methylphenidate. The Incremental Cost-Effectiveness Ratio (ICER) for dexamphetamine is 2,579 €/DALY saved, and 9,437 €/ DALY saved for methylphenidate. The study by Donnelly et al., (2004) used a meta-analysis of randomized controlled trials to derive utility values [33]. In addition, Narayan et al., (2004) found that the costeffectiveness of amphetamine /dextroamphetamine treatment versus none is 19,433 €/DALY within a social perspective. The methods include a decision-tree analysis and the assumption that treatment decision was finalized for each patient within six months [34].
Swedish data provides a trend of costs over time for atomoxetine. At baseline, non-medication costs for the atomoxetine group (combined with parental psycho education) were 828 €, which decreased to 224 € 49 weeks after baseline. Similar results were found for patients from the placebo group who switched to atomoxetine. The cost reduction was due to lower direct and indirect costs. Therefore, the non medication costs (eg. fewer visits to health care providers) can be reduced by atomoxetine treatment [35].
Methylphenidate as a main treatment option can be differentiated into long-acting and short-acting formulations. A study from the Netherlands showed that the total cost associated with methylphenidate treatment after 10 years was 15,739 € for the immediate-release version of the drug, and 16,015 € for the longacting Osmotic Release Oral System (OROS) version. It was stated that long-acting medications are a cost-effective treatment for patients with suboptimal treatment success using immediate-release medication [36]. In addition, general studies, which differentiate between short-acting and long-acting medications, but not by drug, report lower healthcare expenditures for short-acting stimulant users (1,746 €) compared to long-acting stimulant users (2,271 €).Therefore, depot agents seem to be associated with higher total treatment costs; however, the reported annual stimulant expenses were lower than those given in other published studies [37].
Off-Label-Use of antipsychotics for ADHD patients
Atypical and typical antipsychotics are known as off-label treatment options for ADHD. For example, risperidone isan atypical antipsychotic used for ADHD patients. Studies have shown that risperidone can reduce ADHD symptoms, especially if specific comorbidities are diagnosed. Data from the USA indicate that ADHD patients, who switched to or extended their existing therapy with atypical antipsychotics, have higher rates of switching and augmentation, greater resource use, and higher total health care costs (5,233 €) compared to ADHD patients who switched to or augmented to non-antipsychotics (3,538 €) like atomoxetine [38]. A more recent study of Sikirica et al., (2014) confirmed these results [39].
Complementary treatment with guanfacine
Adding guanfacine (extended-release) to another stimulant is a complementary treatment strategy. According to an exemplary study conducted in 2010, total costs for stimulant users are 1,581 € compared to 2,254 € for patients on monotherapy. Higher drug costs can partly be compensated for by lower extraneous medical costs. With an ICER of 23,892 €/QALY, guanfacine in addition to stimulants could be considered cost-effective [40]. Further studies using claims data from the USA differentiate Guanfacine Immediate- Release (GIR) from Guanfacine Extended-Release (GRX) in children and adolescents with ADHD. Total healthcare costs were similar for both agents (GIR 3,180 € vs. GRX 2,969 €) during a 6-month study period. However, GXR users show lower rates of therapy adjustment and fewer inpatient and emergency room visits [41].
Potential of persistence for cost reduction
Another dimension of potential reduction of treatment costs is to investigate drug treatment persistence. Persistence in a detailed exemplary study was defined as at least 1 prescription every 3 months during the year after the first methylphenidate prescription. Savings in total costs of 187 € per year could be identified for persistent patients compared to the non-persistent group. Thus, it can be concluded that treatment costs can potentially be reduced if ADHD patients are persistent. The study consisted of German claims data [42].
Behavioral and multimodal treatment
In addition to drug treatment, the costs and efficiency of multimodal treatment of children was evaluated by Jensen et al., (2005). Several treatment options were considered for medication management, as well as behavioral treatment and a combination of both in this study. The main result was that medical management treatment is likely to be more cost-effective in treatment for children with ADHD, particularly in those with lower severity. Cost per treatment arm within 14 months of treatment was 1,280 €, while for intensive behavioral treatment (defined predominantly by a high proportion of parent, school, and child components), mean costs were 7,577 €. Combined treatment with medical management and behavioral treatment was more costly than routine community care (i.e., 8,487 € versus 1,161 €). Medical management is more costly but also more effective than community care. It has been estimated that 390 € has to be invested to treat a child and provide a nearly normal life. An ICER of 32,356 € suggests that it is most cost-effective to use combined treatment for children with comorbid disorders (i.e., internalizing disorders, such as anxiety or depression, and externalizing disorders, such as conduct or oppositional defiant disorder). Costs per additional child treated by combined treatment are higher (ICER of 80,889 €) than the costs of the same treatment for children with ADHD plus both types of comorbid disorder (32,356 €) in comparison to medical management alone. The analyses by Jensen et al., (2005) were derived from the Multimodal Treatment Study of Children with ADHD (MTA) [43]. If a medication strategy fails, a switch to behavioral treatment (2,662 €total costs) should be avoided compared to a switch to another drug (2,209 € total costs) [44].
Because the aim of this review was not only to consolidate existing evidence, but to provide future research ideas, a speculative view on the evidence without specific monetary values for cost reduction is part of the following discussion.
Discussion
Summary of findings
To summarize, the existing evidence shows that medication is a cost-effective treatment strategy compared to no treatment. Additionally, atomoxetine is a cost-effective alternative to methylphenidate [29–31]. The effects of long-acting and short-acting formulations have an influence on cost-effectiveness regarding the patients’ characteristics (e.g., persistent/non-persistent patients) [42]. Furthermore, guanfacine used as a complementary strategy is costeffective and with respect to multimodal treatment, more evidence is necessary to determine the cost-effectiveness. In some cases, costeffectiveness can be assumed [40,43].
Influences on treatment cost-effectiveness
Treatment costs for ADHD could be high if hospitalization is necessary [19]. While only a small number of patients are hospitalized because of ADHD, these cases can be monitored and personal care provided because they are high-cost cases. Secnik et al., (2005) found no significant inpatient cost difference in employees diagnosed with ADHD and their control group in the USA. This is explained by the fact that only a small number of patients are hospitalized due to ADHD [45]. Additionally, Braun et al., (2013) reported that the proportion of costs due to inpatient care was 20%, but that the larger share of the total costs was due to therapeutic devices and remedies (43.8%). Therefore, costs for therapeutic devices and remedies should be recognized for potential treatment cost reduction [46].
In addition, treatment costs for ADHD increase with the number of comorbidities [47,48], such as asthma, anxiety, bipolar disorder, depression, drug or alcohol abuse, and oppositional disorder. The cost associated with patients with this level of comorbidity is significantly higher compared to control groups without ADHD. Consequently, direct and indirect costs associated with ADHD treatment have the potential for cost reduction [45]. It has to be recognized that in the second year after diagnosis, the adjusted excess costs for children with ADHD compared with the costs for children without ADHD are lower than in the first year after diagnosis, but not for patients with comorbidities. A study with data from a nonprofit integrated health care delivery system in California shows a decrease in adjusted health care system costs of 151 € from the first year after index diagnosis, compared to the second year after index diagnosis for patients without comorbidity. However, the findings in the unadjusted data are different in the cohort of ADHD children with coexisting mental health conditions. The influence of comorbidities is evident by the cost increase from the first to the second year of diagnosis (i.e., 280 €) [14]. Therefore, there is an important influence of comorbidities on the cost of illness and their treatment options. For example, diagnostic measures, such as EEG screening before initiating stimulant medication for ADHD, is a cost-effective method for preventing sudden cardiac death [49].
Indirect factors of the most cost-effective medication strategy
In line with other reviews, drug therapy seems to be superior to no medication treatment strategy [23,50]. For example, Catala Lopez et al., (2013) stated that methylphenidate and atomoxetine are cost-effective alternatives compared to placebo or no treatment, but direct comparison methylphenidate and atomoxetine treatment show contradictory results [25]. In ADHD cases with normal course and no comorbidities, pharmacotherapy is superior compared to behavioral treatment only [24]. Furthermore, there is evidence that extendedrelease methylphenidate has a higher compliance rate and less injuries versus more costly 3-times-daily immediate-release methylphenidate. Higher compliance could decrease costs associated with the need for re-evaluation for dose adjustment, to switch medications, and to expand therapy [51]. Other outcome parameters used to estimate cost-effectiveness include emergency room rates, medication switch probability, and periods of persistence (adherence) [52].
Medication side effects, such as abuse liability, cause direct and indirect costs; these, however, have been reduced by the availability of longer-acting agents. A large-scale community survey in the USA has shown that abuse is more common with short-acting preparations [53]. Therefore, long-acting ADHD drugs have nearly replaced shortacting stimulant use for children with ADHD [54].
Pharmacotherapy has shown significant benefits in patients’ outcomes as well as direct and indirect savings, however, drugs are increasingly costly due to their expanding use. Therefore, management of ADHD and promoting adherence are likely to improve cost-effectiveness [55].
Additionally, switching drugs may lead to higher treatment costs due to medication adjustment. Among patients using longacting methylphenidate, the rate of drug switching was the lowest [56]. Furthermore, patient’s out-of-pocket payments can be used to reduce treatment costs because these raise awareness for costs and the need for treatment. Out-of-pocket payments are lower for generic prescriptions than for brand name prescriptions [57]. Thus, price conscious out-of-pocket-payments could reduce treatment costs. However, it may also lead to a lack of health care in population groups with low income.
Guidelines define first choice medication. Methylphenidate is the most common first-line drug but atomoxetine is more effective if certain comorbidities are diagnosed [58]. The discussion about medication or non-pharmaceutical therapy alone is not useful. Optimal treatment should be multimodal. Intensive medication management may be cost-effective for uncomplicated cases, but the resources saved by higher cost-effectiveness should be used for other types of treatment for patients of higher complexity or severity [59].
Innovative treatment options
Other non-pharmaceutical treatment options have implications for cost reduction. These options mainly consist of behavioral training, but also include sport and nutritional medicine. The evidence for alternative treatment compared to pharmaceutical treatment is sparse and, to the best of our knowledge, Jensen et al., (2005) is the only publication that reports the cost-effectiveness of non-medication treatments for ADHD [60]. Even if costs are not the focus of the investigation, an overview of the effectiveness could provide information about best-practice treatment strategies and potential for cost reduction. These studies with no explicit costeffectiveness are presented in the following section. The evidence for effectiveness is higher for parental behavior training than for child training [61]. Psychiatric treatment is often considered effective treatment for ADHD and cost-effective [62]. Similar results are found in the area of behavioral therapy; however, group cognitive behavioral therapy is assumed cost-effective [63]. Another treatment strategy for ADHD is neuro feedback. One study showed that neuro feedback could achieve a high cost-benefit ratio. In contrast to drug treatment, the causes for ADHD, not the symptoms, are addressed. The use of neuro feedback is supposed to reduce medication use and the learned behavior is presumed to endure over 10 years. Furthermore, neuro feedback is not associated with side effects [64,65]. More, controlled studies are necessary to provide these messages as true. In addition, the cost-effectiveness of neuro feedback needs to be further explored. Research on the impact of neuropsychological assessment on the psychological, social, academic, and functional wellbeing of ADHD patients is needed [66].
Complementary and alternative treatment options for ADHD could have the potential to reduce treatment costs. However, there is no clear evidence for the cost-effectiveness of these treatments. For example, Sinha et al., (2005) stated that modified diet, vitamins and/ or minerals, dietary supplements, aromatherapy, and chiropractic are forms of treatment used in Australia [67]. A notice has to be made that dietary is a possible complementary treatment option only for a small number of patients. Furthermore, sports can serve as a concomitant treatment within a multimodal treatment strategy. However, to the best of our knowledge, there are no studies on the cost-effectiveness of these treatment strategies. If effectiveness of these treatment options could be shown in a broader way, e.g. dietary and sports could be implemented with relatively low cost. But without a health economic evaluation this cannot be stated as a generalization [68].
Some influences on costs could be identified within the discussion. Hospitalization, therapeutic devices as well as existing comorbidities can influence costs. But it can also be supposed that compliant patients can provide lower costs over a longer period.
Limitations of included studies and implications for future research
There are some limitations with this review. There may be a risk of bias across the studies as German terms were used, perhaps resulting in a skewed perspective on the topic. However, because all relevant studies published in English were included, the addition of German studies supports the comprehensiveness of our review. Because the majority of the articles report results from USA populations [28,32,34,37,38,40,41,43], the cost information may have been affected since there are substantial differences in global health care systems. This is another reason to include German studies as well as those from other countries. In general, there are limitations in systematic literature reviews. For example, not all of the relevant studies may be included, or heterogeneous studies may be inappropriately combined. Therefore, we implemented a manual literature review and screening of existing reviews. However, there are also limitations regarding the included studies. Markov-models differ in their assumptions as well as other study types as shown within the results section. Within the study of Sikirica et al., (2012) e.g. for the used Markov model some costs for health care status had to be estimated by other studies, but sensitivity analysis showed that the results were not sensitive for the assumptions applied [40]. Chan et al., (2002) reveals a possibility of under identification of ADHD patients by the method (telephone survey). Parents often don’t want to stigmatize their children as ADHD patients. But the authors conducted a validation by medication data and found only a small number of patients with a stimulant medication who were not reported as ADHD patients. This fact suggests that there is no comprehensive limitation by underreporting within the telephone survey [28].
There might be some restriction to estimate the utilities by expert opinion or placebo controlled clinical trial data. Utilities were not collected in a head-to-head clinical trial and not estimated from the patient perspective in two studies [29,30]. The study by Donnelly et al., (2004) used a meta-analysis of randomized controlled trials to derive utility values and raised the awareness that the results might be not representative. They stated that most trials excluded patients with chronic medical or neurological diseases [33]. For the quality of the studies and for comparison with the results of other authors small samples (N=99) [35] regarding the number of patients or included experts (N=5) [36] can be an issue to derive costs. Furthermore, for comparison with other studies it has to mentioned that ICD-9-CM code 314.00 (attention deficit disorder without hyperactivity) was included by Wu et al., (2007), which is not restricted specifically to the ADHD disease [32]. Within German claims data clinical parameters, like severity, were not available and, therefore, no adjustment was provided in this study [42]. A closer examination on the study of Jensen et al., (2005) has to be done as this study is the only one observing cost-effectiveness of behavioral and multimodal treatment. The behavioral interventions utilized in the MTA, which was used as data base for the study, are supposed to be more intensive and lengthy than in community practice. For instance, only children with ADHD combined type (no inattentive patients) were included in this study [43]. Costs associated with behavioral interventions can be higher than what is typically seen in community practice and that affects the comparison to medication treatment. Although, it might bring limitations regarding the transferability to practice, it is the only study which includes cost-effectiveness of behavioral treatment. There is a need to evaluate behavioral treatment more precisely to provide stronger evidence for the treatment strategy.
The differing methodology including the different identification algorithm of ADHD patients can bias comparison of the results. However, the inclusion of different study types broadens the view on the topic and provides a range for cost-effectiveness, which can be interpreted as a more sensitive analysis of the results. Inclusion or exclusion of indirect costs has an influence on total costs as well. Therefore, this perspective is included in (Tables 1 and 2) in order to classify the study. Additionally, the studies included in our review are from a limited time range (2002 to 2013; Tables 1 and 2). Medical practice, legal regulation, and pricing could have changed during this time, which affects cost estimates. Furthermore, all but one article [32] dealt with the cost-effectiveness of ADHD medications in children alone, despite the fact that ADHD is a chronic disorder that in most cases persists into adulthood. In adulthood, rates of comorbidity increase, which affects total cost and the choice of optimal treatment options. Therefore, the age of the included patients should be compared in future studies and reviews. It could be concluded that overall treatment costs are a function of these different components.
Conclusion
This review has shown evidence that medications are cost-effective. Analysis of treatment shows that particular ADHD age groups are supplied only with methylphenidate by approximately one-quarter to one-third [2]. This illustrates that although methylphenidate is a costeffective treatment and predominantly defined as first-line treatment, it is not the main treatment option. However, a lack of information remains regarding behavioral treatment and other complementary measures that could help to reduce treatment costs. Furthermore, research should analyze the cost-effectiveness associated with every stage of ADHD treatment.
It is necessary to evaluate behavioral and pharmacological treatment options in the short-and long-term. In contrast to drug therapy, behavioral treatment efficacy is often found only in the middle-to-long-term period. This must be considered in the assessment of behavioral therapies versus drug therapies. Contrarily, there could be consecutive long-term medication-based symptoms that influence cost-effectiveness [69]. With more evidence limited resources could be better allocated to more cost effective-treatment options.
Acknowledgement
The authors have no relevant affiliations or financial involvement with any organization, which would affect this article. No assistance was utilized during the writing process of this manuscript.
References
- Gebhardt B, Glaeske G. ADHS bei Kindern und Jugendlichen: Befragungsergebnisse und Auswertungen von Daten der Gmünder ErsatzKasse GEK. St. Augustin: Asgard-Verl; 2008.
- Jans T, Kreiker S, Warnke A. [Multimodal treatment of attention-deficit hyperactivity disorder in children]. Nervenarzt. 2008; 79: 791-800.
- Ebert D, Krause J, Roth-Sackenheim C. [ADHD in adulthood--guidelines based on expert consensus with DGPPN support]. Nervenarzt. 2003; 74: 939-946.
- Habler F, Kosters M, Streeck-Fischer A. Hyperkinetische Storungen: Kompendium Adoleszenz psychiatrie: Krankheitsbilder mit CME-Fragen. 2011; 398-426.
- Lopez-Munoz F, Alamo C, Quintero-Gutierrez FJ, García-García P. A bibliometric study of international scientific productivity in attention-deficit hyperactivity disorder covering the period 1980-2005. European child & adolescent psychiatry. 2008; 17: 381-991.
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. International Journal of Epidemiology. 2014.
- Erskine HE, Ferrari AJ, Polanczyk GV, Moffitt TE, Murray CJ, Vos T, et al. The global burden of conduct disorder and attention-deficit/hyperactivity disorder in 2010. J Child Psychol Psychiatry. 2014; 55: 328-336.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington.
- Dilling H. Internationale Klassifikation psychischer Störungen: ICD-10 Kapitel V (F), Klinisch-diagnostische Leitlinien. 9. Ed., unter Berücksichtigung der Änderungen entspr. ICD-10-GM 2014. Bern: Huber; 2014.
- Retz-Junginger P, Sobanski E, Alm B, Retz W, Rösler M. [Age and gender aspects of attention-deficit hyperactivity disorder]. Nervenarzt. 2008; 79: 809-819.
- Schlander M, Trott GE, Schwarz O. [The health economics of attention deficit hyperactivity disorder in Germany. Part 2: Therapeutic options and their cost-effectiveness]. Nervenarzt. 2010; 81: 301-314.
- Hazell P. Review of new compounds available in Australia for the treatment of attention-deficit hyperactivity disorder. Australia Psychiatry. 2004; 12: 369-75.
- Nutt DJ, Fone K, Asherson P, Bramble D, Hill P, Matthews K, et al. Evidence-based guidelines for management of attention-deficit/hyperactivity disorder in adolescents in transition to adult services and in adults: Recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2007; 21: 10-41.
- Ray GT, Levine P, Croen LA, Bokhari FA, Hu TW, Habel LA. Attention-deficit/hyperactivity disorder in children: excess costs before and after initial diagnosis and treatment cost differences by ethnicity. Archives of pediatrics & adolescent medicine. 2006; 160: 1063-1069.
- Romanos M, Schwenck C, Walitza S. [Diagnosis of attention-deficit hyperactivity disorder in childhood and adolescence]. Nervenarzt. 2008; 79: 782-790.
- Gillberg C, Gillberg IC, Rasmussen P, Kadesjö B, Söderström H, Råstam M, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004; 13: I80-92.
- Birnbaum HG, Kessler RC, Lowe SW, Secnik K, Greenberg PE, Leong SA, et al. Costs of Attention Deficit-Hyperactivity Disorder (ADHD) in the US: excess costs of persons with ADHD and their family members in 2000. Current medical research and opinion. 2005; 21: 195-206.
- Doshi JA, Hodgkins P, Kahle J, Sikirica V, Cangelosi MJ, Setyawan J, et al. Economic Impact of Childhood and Adult Attention-Deficit/Hyperactivity Disorder in the United States. Journal of the American Academy of Child & Adolescent Psychiatry. 2012; 51: 990-1002.
- Wehmeier PM, Schacht A, Rothenberger A. Change in the direct cost of treatment for children and adolescents with hyperkinetic disorder in Germany over a period of four years. Child Adolesc Psychiatry Ment Health. 2009; 3: 3.
- Matza LS, Paramore C, Prasad M. A review of the economic burden of ADHD. Cost Eff Resour Alloc. 2005; 3: 5.
- Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Journal of pediatric psychology. 2007; 32: 711-727.
- Schlander M. Impact of Attention-Deficit/Hyperactivity Disorder (ADHD) on prescription dug spending for children and adolescents: increasing relevance of health economic evidence. Child and adolescent psychiatry and mental health. 2007; 1: 13.
- King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al. A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamphetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents. Health technology assessment (Winchester, England). 2006; 10:146.
- Wu EQ, Hodgkins P, Ben-Hamadi R, Setyawan J, Xie J, Sikirica V, Du EX. Cost effectiveness of pharmacotherapies for attention-deficit hyperactivity disorder: a systematic literature review. CNS Drugs. 2012; 26: 581-600.
- Catala-Lopez F, Ridao M, Sanfélix-Gimeno G, Peiró S. Cost-effectiveness of pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: Qualitative synthesis of scientific evidence. Revista de Psiquiatría y Salud Mental (English Edition). 2013; 6: 168–177.
- Madaan V, Daughton J, Lubberstedt B, Mattai A, Vaughan BS, Kratochvil CJ. Assessing the efficacy of treatments for ADHD: overview of methodological issues. CNS Drugs. 2008; 22: 275-290.
- Seixas M, Weiss M, Muller U. Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J Psychopharmacol. 2012; 26: 753-765.
- Chan E, Zhan C, Homer CJ. Health care use and costs for children with attention-deficit/hyperactivity disorder: national estimates from the medical expenditure panel survey. Arch Pediatr Adolesc Med. 2002; 156: 504-511.
- Cottrell S, Tilden D, Robinson P, Bae J, Arellano J, Edgell E, et al. A modeled economic evaluation comparing atomoxetine with stimulant therapy in the treatment of children with attention-deficit/hyperactivity disorder in the United Kingdom. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2008; 11: 376–388.
- Prasad S1, Arellano J, Steer C, Libretto SE. Assessing the value of atomoxetine in treating children and adolescents with ADHD in the UK. Int J Clin Pract. 2009; 63: 1031-1040.
- Hong J, Dilla T, Arellano J. A modelled economic evaluation comparing atomoxetine with methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder in Spain. BMC psychiatry. 2009; 9: 15.
- Wu EQ, Birnbaum HG, Zhang HF, Ivanova JI, Yang E, Mallet D. Health care costs of adults treated for attention-deficit/hyperactivity disorder who received alternative drug therapies. J Manag Care Pharm. 2007; 13: 561-569.
- Donnelly M, Haby MM, Carter R, Andrews G, Vos T. Cost-effectiveness of dexamphetamine and methylphenidate for the treatment of childhood attention deficit hyperactivity disorder. Aust N Z J Psychiatry. 2004; 38: 592-601.
- Narayan S, Hay J. Cost effectiveness of methylphenidate versus AMP/DEX mixed salts for the first-line treatment of ADHD. Expert Rev Pharmacoecon Outcomes Res. 2004; 4: 625-634.
- Myrén KJ, Thernlund G, Nylén A, Schacht A, Svanborg P. Atomoxetine's effect on societal costs in Sweden. J Atten Disord. 2010; 13: 618-628.
- Faber A, Van Agthoven M, Kalverdijk LJ, Tobi H, Jong-van den Berg LT de, Annemans L, et al. Long-acting methylphenidate-OROS in youths with attention-deficit hyperactivity disorder suboptimally controlled with immediate-release methylphenidate: a study of cost effectiveness in The Netherlands. CNS drugs. 2008; 22: 157–170.
- Stevens J, Harman JS, Kelleher KJ. Sociodemographic and economic comparisons of children prescribed longer-acting versus short-acting stimulant medications for attention deficit hyperactivity disorder. The journal of behavioral health services & research. 2005; 32: 430–437.
- Sikirica V, Pliszka SR, Betts KA, Hodgkins P, Samuelson T, Xie J, et al. Comparative treatment patterns, resource utilization, and costs in stimulant-treated children with ADHD who require subsequent pharmacotherapy with atypical antipsychotics versus non-antipsychotics. Journal of managed care pharmacy: JMCP. 2012; 18: 676–689.
- Sikirica V, Pliszka SR, Betts KA, Hodgkins P, Samuelson TM, Xie J, et al. Impact of atypical antipsychotic use among adolescents with attention-deficit/hyperactivity disorder. Am J Manag Care. 2014; 20: 711-721.
- Sikirica V, Haim Erder M, Xie J, Macaulay D, Diener M, Hodgkins P, et al. Cost effectiveness of guanfacine extended release as an adjunctive therapy to a stimulant compared with stimulant monotherapy for the treatment of attention-deficit hyperactivity disorder in children and adolescents. Pharmaco Economics. 2012; 30: 1–15.
- Sikirica V, Xie J, Lizhang He T, Haim Erder M, Hodgkins P, Yang H, et al. Immediate-release versus extended-release guanfacine for treatment of attention-deficit/hyperactivity disorder. Am J Pharm Benefits. 2013; 5: 85-94.
- Braun S, Russo L, Zeidler J, Linder R, Hodgkins P. Descriptive comparison of drug treatment-persistent, -nonpersistent, and nondrug treatment patients with newly diagnosed attention deficit/hyperactivity disorder in Germany. Clinical therapeutics. 2013; 35: 673–685.
- Jensen PS, Garcia JA, Glied S, Crowe M, Foster M, Schlander M, et al. Cost-effectiveness of ADHD treatments: findings from the multimodal treatment study of children with ADHD. The American journal of psychiatry. 2005; 162: 1628–1636.
- Vanoverbeke N, Annemans L, Ingham M, Adriaenssen I. A cost analysis of the management of attention-deficit/hyperactivity disorder (ADHD) in children in the UK. Journal of medical economics. 2003; 6: 79–94.
- Secnik K, Swensen A, Lage MJ. Comorbidities and costs of adult patients diagnosed with attention-deficit hyperactivity disorder. Pharmaco Economics. 2005; 23: 93–102.
- Braun S, Zeidler J, Linder R, Engel S, Verheyen F, Greiner W. Treatment costs of attention deficit hyperactivity disorder in Germany. The European journal of health economics: HEPAC: health economics in prevention and care. 2013; 14: 939–945.
- Guevara J, Lozano P, Wickizer T, Mell L, Gephart H. Utilization and cost of health care services for children with attention-deficit/hyperactivity disorder. Pediatrics. 2001; 108: 71-78.
- Hakkaart-van Roijen L, Zwirs BWC, Bouwmans C, Tan SS, Schulpen TWJ, Vlasveld L, et al. Societal costs and quality of life of children suffering from attention deficient hyperactivity disorder (ADHD). Eur Child Adolesc Psychiatry. 2007; 16: 316–326.
- Denchev P, Kaltman JR, Schoenbaum M, Vitiello B. Modeled economic evaluation of alternative strategies to reduce sudden cardiac death among children treated for attention deficit/hyperactivity disorder. Circulation. 2010; 121: 1329–1337.
- Schlander M. The pharmaceutical economics of child psychiatric drug treatment. Curr Pharm Des. 2010; 16: 2443-2461.
- Lage M, Hwang P. Effect of methylphenidate formulation for attention deficit hyperactivity disorder on patterns and outcomes of treatment. J Child Adolesc Psychopharmacol. 2004; 14: 575–581.
- Kemner JE, Lage MJ. Effect of methylphenidate formulation on treatment patterns and use of emergency room services. Am J Health Syst Pharm. 2006; 63: 317-322.
- Manos MJ, Tom-Revzon C, Bukstein OG, Crismon ML. Changes and challenges: managing ADHD in a fast-paced world. J Manag Care Pharm. 2007; 13: 211-213.
- Fullerton CA, Epstein AM, Frank RG, Normand SL, Fu CX, McGuire TG. Medication use and spending trends among children with ADHD in Florida's Medicaid program, 1996-2005. Psychiatr Serv. 2012; 63: 115-121.
- Dopheide JA1. The role of pharmacotherapy and managed care pharmacy interventions in the treatment of ADHD. Am J Manag Care. 2009; 15: S141-150.
- Sasané R, Hodgkins P, Meijer W. Treatment stabilization in children and adolescents with attention-deficit/hyperactivity disorder: data from the Netherlands. Curr Med Res Opin. 2010; 26: 2565-2574.
- Lenderts S, Kalali AH. Average Out-of-Pocket Expenses Across Different Drug Categories and Commercial Third-Party Payers. Psychiatry (Edgmont). 2010; 7: 12-13.
- Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009; 11: 203-226.
- Foster EM, Jensen PS, Schlander M, Pelham WE Jr, Hechtman L, Arnold LE, et al. Treatment for ADHD: is more complex treatment cost-effective for more complex cases? Health Serv Res. 2007; 42: 165-182.
- Beecham J. Annual Research Review: Child and adolescent mental health interventions: a review of progress in economic studies across different disorders. J Child Psychol Psychiatr. 2014.
- Ghuman JK, Arnold LE, Anthony BJ. Psychopharmacological and other treatments in preschool children with attention-deficit/hyperactivity disorder: current evidence and practice. Journal of child and adolescent psychopharmacology. 2008; 18: 413–447.
- Trott GE. Attention-deficit/hyperactivity disorder (ADHD) in the course of life. European archives of psychiatry and clinical neuroscience. 2006; 256: 21–25.
- Bramham J, Young S, Bickerdike A, Spain D, McCartan D, Xenitidis K. Evaluation of group cognitive behavioral therapy for adults with ADHD. J Atten Disord. 2009; 12: 434-441.
- Butnik SM. Neuro feedback in adolescents and adults with attention deficit hyperactivity disorder. J Clin Psychol. 2005; 61: 621-625.
- Pop-Jordanova N, Markovska-Simoska S, Zorcec T. Neuro feedback treatment of children with attention deficit hyperactivity disorder. Prilozi / Makedonska akademija na naukite i umetnostite, Oddelenie za bioloski i medicinski nauki = Contributions / Macedonian Academy of Sciences and Arts, Section of Biological and Medical Sciences. 2005; 26: 71–80.
- Pritchard AE, Nigro CA, Jacobson LA, Mahone EM. The role of neuropsychological assessment in the functional outcomes of children with ADHD. Neuropsychol Rev. 2012; 22: 54-68.
- Sinha D, Efron D. Complementary and alternative medicine use in children with attention deficit hyperactivity disorder. J Paediatr Child Health. 2005; 41: 23-26.
- Leithauser R. / Beneke R. Sport bei ADHS - Plan fur Desaster Oder verschenkte Resource? Deutsche Zeitschrift für Sportmeidzin. 2013; 64.
- Schlander M. Long-acting medications for the hyperkinetic disorders. A note on cost-effectiveness. Eur Child Adolesc Psychiatry. 2007; 16: 421-429.