
Research Article
Austin J Psychiatry Behav Sci. 2016; 3(2): 1052.
Gender Differences in Vitamin D and Depressive Symptoms: A Systematic Review
Day CMJ¹*, Kripalani M¹, Rees K², Weich S², Clarke A² and Stranges S³
¹South London and Maudsley NHS Trust, University Hospital Lewisham, UK
²Division of Health Sciences, University of Warwick Medical School, UK
³Department of Population Health, Luxembourg Institute of Health, Luxembourg
*Corresponding author: Day CMJ, Lady well Unit, South London and Maudsley NHS Trust, University Hospital Lewisham, London, UK
Received: June 02, 2016; Accepted: July 08, 2016; Published: July 12, 2016
Abstract
Background: Both vitamin D insufficiency and depression are major public health concerns particularly in women. Recent animal and cross-sectional studies have suggested an association between low vitamin D levels and increased depressive symptoms with some evidence that vitamin D supplementation may be more beneficial in women.
Objective: (i) To examine the gender differences in the observational prospective association between vitamin D status (using serum levels) and depressive symptoms; (ii) to determine if vitamin D supplementation is more effective in women than in men.
Methods: MEDLINE, EMBASE, Web of Science & Knowledge, PsychInfo, the Cochrane Library and trial registers were searched with no language restrictions from inception to December 2015.
Results: 22 separate studies met the inclusion criteria, 5 observational prospective studies and 17 RCTs. The most robust cohort study found a significant association only in women. Only seven out of seventeen RCTs found a significant effect of vitamin D on depressive symptoms. Six of these were mixed-sex studies and one was a female only study that had the greatest effect size. The females included in these seven trials were mostly of childbearing age.
Conclusion: There is suggestive evidence of potential benefits of vitamin D supplementation in women of childbearing age with depressive symptoms. Further large randomized placebo-controlled secondary prevention trials in both men and women of childbearing age, with low vitamin D levels, are needed to investigate the relative efficacy of vitamin D supplementation in depression in men and women.
Keywords: Systematic review; Vitamin D; Depression; Gender differences; Randomized control studies; Observational studies; Prevention; Vitamin D; Gender differences; Female
Abbreviations
RCT: Randomized Control Trial; BDI: Beck Depression Inventory; CES-D: Centre for Epidemiologic Studies –Depression Scale; SCID: Structured Clinical Interview for DSM Disorders; CIDI: Composite International Diagnostic Interview; NOS: Newcastle-Ottawa Quality Assessment Scale; ADL: Activities of Daily Living; SPPB: Short Physical Performance Battery; PTH: Parathyroid Hormone; HDRS: Hamilton Depression Rating Scale; POMS: Profile of Mood States; ANCOVA: Analysis of Covariance; SF-12 MCS: Mental Component of the Short Form 12 Health Survey; HADS: Hospital Anxiety and Depression Scale; MADRS: Montgomery-Asberg Depression Rating Scale; GDS: Geriatric Depression Scale.
Introduction
Depression is the third most common cause of disease burden in the world for both men and women and is the leading cause of disease burden for women in the world [1]. Epidemiological data show that women are twice as likely as men, to develop depression during their childbearing years [2-4]. Furthermore, it is estimated that 1 billion people worldwide have either deficient or insufficient vitamin D levels [5], and some epidemiological data show that women may be more at risk of this than men [6-13] women. Vitamin D fortification of milk was mainstream in Europe in the early twentieth century; however this was banned in the 1950’s due to case reports of hypercalcaemia in children [5].
For both sexes, there is now epidemiological evidence of association of hypovitaminosis D with many non-skeletal health conditions [5,14] and more recently, cross-sectional and cohort studies have shown an association between low vitamin D levels and depression [15-17]. These epidemiological findings are corroborated by biological experimental evidence supporting a potential role of vitamin D in the pathogenesis of depression [18-20].
Given that women are at increased risk of depression and hypovitamonis D, there has already been some interest in the role of vitamin D in depression in women [21]. Some cross-sectional data show that the association between low vitamin D levels and depressive symptoms is stronger in women than in men [22,23]. It is not clear whether this is because women are, a priori, more at risk of hypovitaminosis D or whether there is a specific genderbased interaction between vitamin D and depression. Like the sex hormones, active vitamin D is a steroid hormone and there is evidence that oestrogen promotes the potency of vitamin D protective effects in animals and humans [24, 25].
One existing systematic review and meta-analysis [26] reported a modest significant effect of vitamin D supplementation on depressive symptoms in adults with depressive symptoms. Another metaanalysis [27] after eliminating studies with biological flaws also found benefit of vitamin D supplementation. In this systematic review, however we aim to investigate any sex-interaction in the association between vitamin D levels and depressive symptoms and in the effect of vitamin D supplementation on depressive symptoms, which has not been explored yet. From current evidence of micronutrient supplementation trials, where, supplementation is more effective in high-risk individuals [28], we hypothesise that the effect of vitamin D supplementation will be most clearly seen in (a) those with depressive symptoms at baseline of both sexes and (b) those who are vitamin D deficient. Furthermore, given the oestrogen promoted differences in local vitamin D metabolism, we hypothesize that both effects (a) and (b) will be greater in women than in men.
Materials and Methods
Search strategy and study selection
The following electronic databases were systematically searched with no language restrictions from their inception to December 2015; In MEDLINE; EMBASE; Web of Science; Web of knowledge; Web of Knowledge and Science (title); Psych Info; Cochrane Library and Meta RCT trial registers. Existing reviews and articles retrieved from the search terms were scanned for further studies and hand searching of various relevant journals was also performed. Due to the diverse nomenclature of vitamin D, extensive search strategies were created and tailored to individual databases. The search strategy for the various databases is shown in the appendix.
Studies met the inclusion criteria if they fulfilled the following criteria:
- Study design - observational prospective studies (cohort/ nested case control studies) or Randomized Control Trials (RCTs).
- Participants - Adults > 18 years free of depressive disorder at baseline for prospective observational studies and primary prevention trials and with a diagnosis of depressive disorder (either clinically or from validated depression/mood scales) for secondary prevention trials. Those with pregnancy, kidney, thyroid or parathyroid disease were excluded.
- Exposure/intervention – 25-hydroxyvitamin D3 serum levels or any vitamin D supplementation (including multivitamin compound containing vitamin D).
- Outcomes - change in depression/mood scores on a validated, standardized measure, diagnosis or no diagnosis of depressive disorder based on a clinical diagnosis, or a validated, standardized measure (e.g. BDI or CES-D etc.) or interview (e.g. SCID or CIDI).
- World Health Organization. World Health Organization: Global Burden of Disease Report. 2004.
- Williams JB, Spitzer RL, Linzer M, Kroenke K, Hahn SR, DeGruy FV, et al. Gender differences in depression in primary care. Obstet Gynecol. 1995; 173: 654-659.
- Noble RE. Depression in women. Metab Clin Exp. 2005; 54: 49-52.
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu H-G, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996; 276: 293-299.
- Holick MF. Vitamin D Deficiency. N Engl J Med. 2007; 357: 266-281.
- Hoogendijk WJG, Lips P, Dik MG, Deeg DJH, Beekman ATF, Penninx BWJH. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Archives of General Psychiatry. 2008; 65: 508-512.
- Mithal A, Wahl D, Bonjour J, Burckhardt P, Dawson-Hughes B, Eisman JA et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporosis Int. 2009; 20: 1807-1820.
- Van der Wielen, Reggy PJ, De Groot L, Van Staveren W, Lowik MRH, Van den Berg H et al. Serum vitamin D concentrations among elderly people in Europe. The Lancet 1995; 346: 207-210.
- Dawson-Hughes B, Harris SS, Dallal GE. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr. 1997; 65: 67-71.
- Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010; 3: 29.
- Carnevale V, Modoni S, Pileri M, Di Giorgio A, Chiodini I, Minisola S, et al. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: seasonal and gender differences. Osteoporosis Int. 2001; 12: 1026-1030.
- Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, et al. The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr. 2007; 86: 959-964.
- Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007; 85: 860-868.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clinic Proceedings. 2006; 81: 353-373.
- Bertone-Johnson ER. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutrition Reviews. 2009; 67: 481-492.
- Berk M, Sanders KM, Pasco JA, Jacka FN, Williams LJ, Hayles AL, et al. Vitamin D deficiency may play a role in depression. Medical Hypotheses. 2007; 69: 1316-1319.
- Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013; 202: 100-107.
- Fernandes de Abreu DA, Eyles D, Feron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009; 34: 265-277.
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1a-hydroxylase in human brain. J Chem Neuroanat. 2005; 29: 21-30.
- Wang T, Tavera-Mendoza LE, Laperriere D, Libby E, Burton Metal. Large-scale in silico and microarray-based identification of direct 1, 25-dihydroxyvitamin D3 target genes. Molecular endocrinology. 2005; 19: 2685-2695.
- Murphy PK, Wagner CL. Vitamin D and Mood Disorders Among Women: An Integrative Review. Journal of Midwifery and Women's Health. 2008; 53: 440-446.
- Toffanello ED, Sergi G, Veronese N, Perissinotto E, Zambon S, Coin A, et al. Serum 25-hydroxyvitamin d and the onset of late-life depressive mood in older men and women: the pro.v.a. study. J Gerontol A Biol Sci Med Sci, 2014; 69: 1554-1561.
- Kjærgaard M, Joakimsen R, Jorde R. Low serum 25-hydroxyvitamin D levels are associated with depression in an adult Norwegian population. Psychiatry Res. 2011; 190: 221-225.
- Correale J, Ysrraelit MC, Gaitan MI. Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol. 2010; 185: 4948-4958.
- Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005; 175: 4119-4126.
- Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, et al. Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials. Psychosom Med. 2014; 76: 190-196.
- Spedding S. Vitamin D and Depression: A Systematic Review and Meta-Analysis Comparing Studies with and without Biological Flaws. Nutrients. 2014; 6: 1501-1518.
- Guallar E, Stranges S, Mulrow C, Appel LJ, Miller ER. Enough is enough: stop wasting money on vitamin and mineral supplements. Ann Intern Med. 2013; 159: 850-851.
- Wells G, Shea B, O connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. 2000.
- Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008; 88: 491-499.
- Williams J, Houston D, Davis C, Cauley J, Rubin S, Tylavsky F, et al. Vitamin D Status and Depression in Community-Dwelling Older Adults. American Geriatric Society. 2011.
- May HT, Bair TL, Lappe DL, Anderson JL, Horne BD, Carlquist FJ, et al. Association of vitamin D levels with incident depression among a general cardiovascular population. American Heart Journal. 2010; 159: 1037-1043.
- Milaneschi Y, Shardell M, Corsi AM, Vazzana R, Bandinelli S, Guralnik JM, et al. Serum 25-hydroxyvitamin D and depressive symptoms in older women and men. Journal of Clinical Endocrinology & Metabolism. 2010; 95: 3225-3233.
- Yue W, Xiang L, Zhang Y, Ji Y, Li X. Association of Serum 25-Hydroxyvitamin D with Symptoms of Depression after 6 Months in Stroke Patients. Neurochem Res. 2014; 39: 2218-2224.
- Chan R, Chan D, Woo J, Ohisson C, Mellstrom D, Kwok T, et al. Association between serum 25-hydroxyvitamin D and psychological health in older Chinese men in a cohort study. J Affect Disord. 2011; 130: 251-259.
- Bertone-Johnson ER, Powers SI, Spangler L, Larson J, Michael YL, Millen, AE et al. Vitamin D Supplementation and Depression in the Women,Aos Health Initiative Calcium and Vitamin D Trial. American Journal of Epidemiology. 2012; 176: 1-13.
- Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. 2012; 19: 697-703.
- Sanders KM, Stuart AL, Williamson EJ, Jacka FN, Dodd S, Nicholson G, et al. Annual high-dose vitamin D3 and mental well-being: randomised controlled trial. Br J Psychiatry. 2011; 198: 357-364.
- Dumville JC, Miles JNV, Porthouse J, Cockayne S, Saxon L, King C. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. Journal of Nutrition Health and Aging. 2006; 10: 151-153.
- Harris PA, Scott KW, Lightner C, Hassan N, Pulley J, Edwards T, et al. Clinical and Translational Science. Conference. 2010.
- Haskell CF, Robertson B, Jones E, Forster J, Jones R, Wilde A, et al. Effects of a multi-vitamin/mineral supplement on cognitive function and fatigue during extended multi-tasking. Human Psychopharmacology-Clinical and Experimental. 2010; 25: 448-461.
- Brown MA, Goldstein-Shirley J, Robinson J and Casey S. The effects of a multi-modal intervention trial of light, exercise, and vitamins on women's mood. Women & Health. 2001; 34: 93-112.
- Wepner F, Scheuer R, Schuetz-Wieser B, Machacek P, Pieler-Bruha E, Cross HS, et al. Effects of vitamin D on patients with fibromyalgia syndrome: A randomized placebo-controlled trial. PAIN®. 2014; 155: 261-268.
- Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. Journal of Internal Medicine. 2008; 264: 599-609.
- Gariballa S, Forster S. Effects of dietary supplements on depressive symptoms in older patients: A randomised double-blind placebo-controlled trial. Clinical Nutrition. 2007; 26: 545-551.
- Dean AJ, Bellgrove MA, Hall T, Phan WMJ, Eyles DW, Kvaskoff, D et al. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults–a randomised controlled trial. PLoS One. 2011; 6: 25966.
- Arasteh K. A beneficial effect of calcium intake on mood - Experiment 1. Journal of Orthomolecular Medicine. 1994; 9: 199-204.
- Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology. 1998; 135: 319-323.
- Mozaffari-Khosravi H, Nabizade L, Yassini-Ardakani SM, Hadinedoushan H, Barzegar K. The effect of 2 different single injections of high dose of vitamin D on improving the depression in depressed patients with vitamin D deficiency: a randomized clinical trial. J Clin Psychopharmacol. 2013; 33: 378-385.
- Khoraminya N, Tehrani-Doost M, Jazayeri S, Hosseini A, Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust N Z J Psychiatry. 2013; 47: 271-275.
- Kjaergaard M, Waterloo K, Wang CE, Almas B, Figenschau Y, Hutchinson MS, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012; 201: 360-368.
- Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatry Research. 1993; 49: 77-87.
- Pearce S, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ. 2010; 340: 142-147.
- Sneve M, Figenschau Y, Jorde R. Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur J Endocrinol. 2008; 159: 675-684.
- Gariballa S, Forster S, Walters S, Powers H. A randomized, double-blind, placebo-controlled trial of nutritional supplementation during acute illness. Am J Med 2006; 119: 693-699.
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. TRENDS in Endocrinology & Metabolism. 2002; 13: 100-105.
- Cass WA, Smith MP, Peters LE. Calcitriol Protects against the Dopamine-and Serotonin-Depleting Effects of Neurotoxic Doses of Methamphetamine. Ann N Y Acad Sci. 2006; 1074: 261-271.
- Feron F, Burne THJ, Brown J, Smith E, McGrath JJ, Mackay-Sim A, et al. Developmental Vitamin D< sub> 3 deficiency alters the adult rat brain. Brain Res Bull. 2005; 65:141-148.
- Kuningas M, Mooijaart SP, Jolles J, Slagboom PE, Westendorp RGJ, Van Heemst D. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiology of Aging. 2009; 30: 466-473.
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in chronic major and double depression. J Affect Disord. 2000; 60: 1-11.
- Zender R, Olshansky E. Women's mental health: depression and anxiety. Nurs Clin North Am. 2009; 44: 355-364.
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002; 57: 357-384.
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen–serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998; 44: 839-850.
- Young EA. Sex differences and the HPA axis: implications for psychiatric disease. J Gend Specif Med. 1998; 1: 21-27.
- Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005; 4: 43-58.
- Dimmock PW, Wyatt KM, Jones PW, O’Brien PM. Efficacy of selective serotonin-reuptake inhibitors in premenstrual lancet syndrome: a systematic review. 2000; 356: 1131-1136.
- Hagenau T, Vest R, Gissel T, Poulsen CS, Erlandsen M, Mosekilde L, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int. 2009; 20: 133-140.
- Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009; 29: 3713-3720.
- Langlois K, Greene-Finestone L, Little J, Hidiroglou N, Whiting S. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. 2010.
- Nashold FE, Spach KM, Spanier JA, Hayes CE. Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol. 2009; 183: 3672-3681.
- Aarskog D, Aksnes L, Markestad T, Rodland O. Effect of Estrogen on Vitamin D Metabolism in Tall Girls. The Journal of Clinical Endocrinology & Metabolism. 1983; 57: 1155-1158.
After database searching, one author independently screened the titles and abstracts for potential relevance. Following this initial screening, full reports of short-listed studies were obtained and two of the study authors (CD & KR) independently assessed the studies for inclusion using the pre-specified inclusion criteria outlined above. If there was any disagreement over study inclusion a third author was consulted (SS).
Methodological quality
CD and KR independently assessed methodological quality by examining the method of randomization, allocation concealment, blinding and losses to follow-up for RCTs and by using the Newcastle- Ottawa Quality Assessment Scale to assess the quality of the cohort studies [29].
Analysis
Cohort studies
We planned to pool the adjusted effect estimate of developing depression for those with vitamin deficiency (<50nmol/L), vitamin D insufficiency (<75nmol/L) and sufficient vitamin D levels (>75nmol/L) in a meta-analysis 30.
Randomized control trials
We planned to pool the standardized mean changes in depression scores of participants who received vitamin D supplementation and of those who received placebo.
Results
Study selection
Searching electronic databases yielded 2097 references. (Figure 1) describes the flow of studies through the review. 1823 references were excluded after removal of duplicates and initial screening for relevance. Of the remaining 274 studies, 206 were excluded as they were reviews, animal studies, or cross-sectional studies or had irrelevant content, and 22 studies met the inclusion criteria. No ongoing trials were identified on the Meta RCT trial register. No further studies were identified by scanning the reference lists of relevant studies or by the performing citation searches of the 22 included studies.
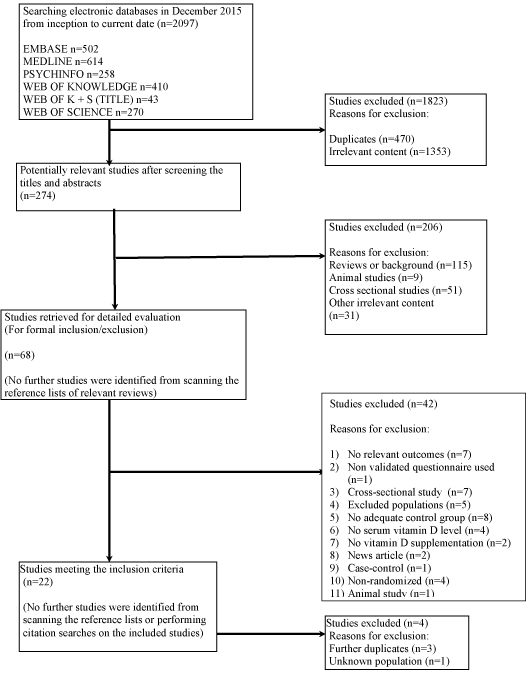
Figure 1: Flow diagram of study selection.
Study characteristics
Details of the included studies are found in (Tables 1-5). Five cohort studies and seventeen RCTs met the inclusion criteria. Given the different populations, interventions, controls and outcomes it was inappropriate to pool the data statistically and so a narrative synthesis was performed.
Cohort Studies: Details of the cohort study characteristics are found in Supplementary (Table 1). All five cohort studies involved older adults without a history of depression; four were in both males and females [31-34] and one included only males [35]. For the exposure, the studies used serum 25-hydroxyvitamin D3 levels split into either quartiles [32,35], tertiles [33], ‘deficient’ (<20ng/ml (50nmol/ml) and ‘sufficient’ (>20ng/ml) levels [31] or levels<28nmol/L vs. >28nmol/L [34]. Three studies measured depression using well-validated depression scales, [31,33,35] one study measured depression by clinical diagnosis [32] and another by clinical diagnosis using a diagnostic algorithm [34]. Follow up periods ranged from 6 months [34], 1 year [32], 3 years [31], 4 years [35] and 6 years [33].
Study, Year
Country
Participants
No
% Female
Age, y
Vitamin D level Exposure definition (nmol/L)
Follow up
Outcome measure
Association between vitamin D levels and depression (adjusted odds and hazard ratios)
Yue et al. 2014 [34]
China
Acute ischaemic stroke patients
184
35.3
62.8 (8.1)
1)<28
2)>28
6 months
Prevalent depression diagnosed according to DSM-III-R criteria using algorithms based on psychiatric interview.
Adjusted Odds ratio
<28nmol/L vs..>28nmol/L: OR 10.32, 95% CI 4.97-28.63; p<0.001
Milan-eschi et al. 2010 [33]
Italy
Healthy adults
74.6% females and 50.4% males had vitamin D < 50nmol/L.
535
55.7
>65
Mean: 73.6
1) <31.7
2) 31.7-53.9
3) >53.9
And
1) <50
2) >50
3 years
6 years
Incident depression diagnosed by Center for Epidemiological Studies Depression Scale (CES-D) (score>16 = depression).
43.6% of females & 20.5% of males developed depressed mood.
Hazard Ratios categorized by tertiles
Women:
Tertile 1 (<31.7nmol/l) vs. Tertile 3 (>53.9nmol/l): HR=2.90(1.53-5.50) p=0.001
Men:
Tertile 1 (<31.7nmol/l) vs. Tertile 3 (>53.9nmol/l): HR=1.67 (0.76-3.68) p=0.21
Hazard Ratios categorized by conventional cut-offs
Women:
(<50nmol/l) vs. (>50nmol/l) HR=2.09(1.25-3.49) p=0.005
Men:
(<50nmol/l) vs. (>50nmol/l) HR=1.46 (0.81-2.65) p=0.21
Fully adjusted Cox regression model did not find statistically significant vitamin D status-by-sex interaction.
Williams et al. 2011 [31]
USA
Well- functioning community dwelling adults.
2240
52%
70-79
1) <50
2) >75
3 years
Incident depression as defined by CES-D score (short) >10 or current treatment with antidepressant medication.
Hazard Ratios (95% CI)
Sufficient vitamin D levels (>75nmol/L) HR: 1
‘Deficient’ vitamin D levels (<50nmol/L) HR: 1.54 (1.19-1.99)
May et al. 2010 [32]
USA
Patients with previous cardio-vascular diagnosis
7358
58.8%
>50
Mean: 73
1) <37.5
2) 40-75
3) 77.5-125
4) >125
1 year
Incident depression defined as having received a clinical diagnosis of depression (ICD-9 codes 296.2-296.36 and 311).
Hazard Ratios (95% CI):
Optimal (>50ng/ml) or ( >125nmol/l); HR = 1
Normal (31-50ng/ml); HR = 1.95 (0.99-3.87) p = 0.06
Low (16-30ng/ml); HR = 2.15 (1.1-4.21) p = 0.03
VL (<15ng/ml); HR = 2.7 (1.35-5.4) p = 0.005
Males only (n=3034): (<<37.5nmol/l) vs. >125nmol/L) HR 6.68, p=0.07
Females only (n=4324): (<37.5nmol/l) vs. >125nmol/L) HR 2.13, p=0.05
No significant interaction between sex and vitamin D (p interaction =0.75).
Chan et al. 2011 [35]
Hong Kong
Chinese community-dwelling male volunteers
801
0%
>65
1) <50
2) 50-74
3) 75-99
4) >100
4 years
Depression diagnosed by face-to-face interviews using a validated Chinese version of Geriatric Depression Scale. (GDS>=8 = diagnosis of depression)
Cases/controls at 4years + Odds Ratios (95% CI)
(50-74nmol/L) = 10/251 OR 95% CI = 1.5(0.16-14.56)
(75-99nmol/L) = 9/234 OR 95% CI = 1.27 (0.13-12.89)
Table 1: Cohort Studies investigating association between vitamin D levels and subsequent depressive symptoms.
Randomized control trials: The study characteristics of the female-only RCTs and mixed sex RCTs are found in Supplementary (Tables 2,3) respectively. Seven out of fourteen RCTs recruited only female participants [36-42]. Five of these were in postmenopausal women, [36-40]. Mixed sex trials included fibromyalgia patients [43], obese & overweight subjects [44], acutely ill elderly hospitalized patients [45] and healthy students [46-49].
Study Year
Country
Participants
Baseline vitamin D levels (nmol/L)
Baseline depressive symptoms
Intervention, n
Control, n
Age, y
Form of Intervention
Dose of Vitamin D
Frequency
Duration
Control
Outcome measure
Summary of Effect
Bertone-Johnson et al. 2012 [36]
USA
Postmenopausal
Subset Mean =52.0+/- 21.1 (n=898)
9.4% (n=212) clinically depressed based on measure
1109
1143
50-79
D3 + calcium tablet
400IU
Daily
2 years
Placebo tablet
Burnam 8-item scale
No effect
Sanders et al. 2011 [38]
Australia
Risk factor for hip fracture or at high risk for hypovitamonisis D
Subset Mean = 49 (n=118)
14.8% (n=298) on antidepressants
1001
1011
>70
D3 tablet
500,000IU
Yearly
3-5 years
Placebo tablet
SF-12 MCS
Trend effect for those on antidepressants at baseline
Dumville et al. 2006 [39]
UK
Risk factor for hip fracture
Not reported
Not reported
689
941
>70
D3 + calcium tablet
800IU
Daily
6 months
Information Sheet
SF-12 MCS
No effect
Yalamanchili & Gallagher 2012 [37]
USA
Postmenopausal community-dwelling
Mean = 76.3 +-9.4
Mean GDS score: 4.8 (4.6) Chronic illnesses excluded. 12% (n=57) clinically depressed based GDS
123
123
65-77
Calcitriol tablet
0.5g
Daily
3 years
Placebo tablet
GDS
No effect, including subanalysis in only those depressed.
Harris & Dawson-Hughes 1993 [53]
USA
Postmenopausal
Not reported
7% (n=18)history of depression
125
125
43-72
D3 + calcium tablet
400IU
Daily
1 year
Placebo tablet
POMS
No effect
Haskell et al. 2010 [41]
UK
Healthy with occasional subjective fatigue
Not measured
Baseline POMS score: 72. Any medically significant diagnosis in last 5 yrs excluded.
100
107
25-50
D3 in multivitamin tablet
200IU
Daily
9 weeks
Placebo tablet
POMS
No effect
106
110
Mean 36
SF-36 MCS
Brown et al. 2001 [42]
USA
Ethnic Minority
Not measured
Mild-Moderate Depressive Symptoms
53
51
>18
D3 in multivitamin tablet + educational session + daily exercise + coach
400IU
Daily
8 weeks
Placebo tablet + educational session + coach
CES-D
Significant effect
POMS
SF-12 MCS: Mental Component Score; GDS: Geriatric Depression Questionnaire; POMS: Profile of Mood States Questionnaire; SF-36 MCS: Mental Component Score; CES-D, Centre for Epidemiology Studies Depression Scale.
Table 2: Female only randomised control trial characteristics and summary of effect of vitamin D supplementation on depressive symptoms.
Country
Participants
Sex
(F:M)
% F
Baseline vitamin D levels (nmol/L)
Baseline depressive symptoms
Interven-tion,n
Control, n
Age, y
Form of intervention
Vitamin D dose
Frequency
Duration
Control
Outcome measure
Effect
Wepner et al. 2014 [43]
Austria
Fibro-myalgia
27:3
90%
<80
Median: 52.1
Range: 21.25-72.5
Not stated
15
15
35-55
Mean 48.37
D3 solution
2400IU or 1200IU
Daily
6 months
Placebo solution
HADS
No effect
Mozaffari-Khosravi et al. 2013 [50]
Iran
Depressive disorder + low vitamin D levels
78:31
72%
<40
Mean BDI-II: 26.9 (7.2)
80
40
20-60
Mean 31.5
D3 injection
Either 150,000 or 300,000IU
Once
n/a
No injection
BDI-II
Significant effect
Khoraminya et al. 2013 [51]
Iran
Major depressive disorder (MDD)
34:6
85%
Mean=58.25
95% patients < 75
MDD: 100% (as per DSM-IV criteria and HDRS>15)
20
20
18-65
Mean 39
D3 tablet plus fluoxetine capsule
1500IU
Daily
8 weeks
Placebo tablet + fluoxetine capsule
HDRS
BDI
Significant effect
Jorde et al.
2008 [44]
Norway
Overweight & obese adults
282:159
64%
Median=52.6
Mean baseline BDI score = 4.5 (Range 0-18)
Antidepressant use excluded.
292
149
21-70
Mean 47.5
D3 capsule + calcium tablet
Either 40,000 IU or 20, 000IU
Weekly
1 year
Placebo + calcium tablet
BDI
Significant effect.In BDI 1-13 effect most clearly seen in females.
Kjaer-gaardeet al. 2012 [52]
Norway
Low vitamin D levels <55nmol/l
129:101
56% F
Mean=47.6 +- 15.7
Those with severe depression excluded. Mean Baseline BDI II scores = 4
Range = 0-49
120
110
30-75
Mean 53.35
D3 capsules
40,000IU
Weekly
6 months
Placebo capsules
BDI II, HADS, MADRS
No overall effect or when stratified to gender. Significant effect seen in those with high BDI, HADS, MADRS score at baseline.
Gariballa & Forster 2007 [45]
UK
Hospitalised patients
83:142
37% F
Not measured
Severe psychiatric history excluded.
37% mild or severe depression (based on GDS).
106
119
>65
Mean 75.6
D3 in multivitamin & nutritional supplement solution
200IU
Daily
6 weeks
Placebo solution
GDS
Significant overall effect after adjustment for baseline GDS, age & gender.
Dean et al. 2011
Australia
Healthy adults
73: 55
57% F
Mean = 76.2 +- 2.6
Current or depressive disorder excluded. Baseline BDI scores = 6.5
63
65
>18
Mean 21.8
D3 capsule
5000 IU
Daily
6 weeks
Placebo capsule
BDI
No effect
Arasteh 2
1994 [47]
USA
Healthy students
Not stated
Not measured
Previous depressive diagnosis excluded. 17% (n=13) ‘depressed’ at baseline based on BDI>9.
76 in total
Not stated
D3 and calcium tablet
1200IU
Daily
4 weeks
Placebo tablet
BDI
Significant effect. Effect increased significantly in those ‘depressed’ at baseline.
Arasteh 1
1994 [48]
USA
Healthy students
Not stated
Not measured
Previous depressive diagnosis excluded. 15% (n=7) ‘depressed’ at baseline based on BDI >9
47 in total
Not stated
D3 and calcium tablet
1200IU
Daily
4 weeks
Placebo tablet
BDI
Significant effect
Lans-downe & Provost 1998 [49]
UK
Healthy students
34:10
77% F
Not measured
Not reported
44 in total.
18-43
Mean 22
D3 and vitamin A capsule
400IU or 800IU
Daily
5 days
Vitamin A capsule
PANAS (Negative affect)
No effect
BDI, Beck Depression Inventory (BDI); BDI II, Beck Depression Inventory II; HADS, Hospital Anxiety & Depression Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; GDS, Geriatric Depression Scale; PANAS, Positive and Negative Affect Schedule.
Table 3: Mixed sex randomized control trial characteristics and summary of effect of vitamin D supplementation on depressive symptoms.
Study
Selection
Comparability
Outcome
Yue 2014 [34]
* *
* *
*
Chan 2011 [35]
* * *
* *
Williams 2011 [31]
* * * *
* *
*
May 2010 [32]
* *
*
Milaneschi 2010 [33]
* * * *
* *
* *
The Newcastle-Ottawa Quality Assessment Scale (NOS) for cohort studies - A study can be awarded a maximum of 4 stars for selection (4 questions relate to the representativeness of the cohort, the selection of the unexposed cohort, ascertainment of exposure and demonstration that the outcome of interest was not present at the start of the study), a maximum of 2 stars for comparability (exposed and unexposed individuals must be matched in the design and/or confounders must be adjusted for in the analysis) and a maximum of 3 stars for outcome (assessment of outcome, follow-up long enough for outcomes to occur, losses to follow-up).
Table 4: Newcastle-Ottawa Quality Assessment Scale (NOS) for cohort studies.
Study
Randomization
Allocation concealment
Blinding
Losses to follow-up
Khoraminya et al. 2013 [51]
‘Randomly assigned’
Unclear
Study states double blind, placebo-controlled but not explicitly described.
Intervention: 4.8%
Control: 4.8%
Wepner et al. 2014 [43]
Random sampling performed by statistician using STATISTICA 7 software (uniform random number generator) was used b.
Unclear
Double blind stated – although patients and doctors in charge of treatment were aware of what vitamin D dose they were taking.
Intervention: 37.5%
Control: 17%
Mozaffari-Khosravi et al. 2013 [50]
Random numbers table
Poor
No blinding – control group received no injection.
Control: 15%
Low dose vit D group: 10%
High dose vit D group: 2.5%
Kjaergaard et al. 2012 [52]
Performed by a central randomization unit. Participants with low vitamin D were randomized en block, stratified by gender and smoking status, into vitamin D group and a placebo group.
Central randomization unit personnel did not have contact with study participants. Personnel were informed of participants’ vitamin D status by the doctor who had access to the serum 25(OH)D levels measured in the sixth Troms Ĝ study but who did not have access to participants. Even participants with high vitamin D status who were not to continue in intervention study went through randomization process to ensure study nurses remained masked.
Participants were blinded: Independent pharmacists dispensed either active or placebo capsules, which were pre-packed in boxes and consecutively numbered according to a computer generated randomization list. Each participant was assigned an order number and received the capsules in the corresponding pre-packed box.
Study nurses were blinded.
No mention of assessors: although BDI is self report.
Placebo group = 9%
Vitamin D group = 2%
Dean et al. 2011 [46]
Randomization sequence was generated by external clinical trials site. A varying-block randomization protocol was used.
Two researchers not involved in generating the randomization sequence assigned participants to the next consecutive participant number.
All investigators, outcome assessors & participants were blinded to treatment allocation procedures and treatment group throughout the study.
Intervention=0%
Placebo = 1.5%
Dumville et al. 2006 [39]
‘Randomization’ by computer
Randomization carried out by independent researchers
No placebo - information sheet given on increasing calcium
Intervention = 25%
Control = 22%
Jorde et al. 2008 [44]
Participants were randomized (not stated how) and stratified by gender and smoking status.
Double blind but allocation concealment not mentioned.
Double blind. Placebo capsules had identical appearance to vitamin D capsules. Authors do not state how investigators were blinded.
High dose intervention = 23%
Low dose intervention = 25%
Control = 25%
Lansdowne & Provost 1998 (n<100) [49]
Authors state the dispensation procedure was random and double blind.
Authors state the dispensation procedure was random and double blind. Nil else reported.
‘Double blind’. No description.
None reported
Haskell et al. 2010 [41]
Computer generated randomization schedule provided by manufacture
Study product dispensed according to corresponding designated randomization number.
Participants and investigators both blinded.
SF-36 MCS = 4.4%
POMS = 8.4%
Bertone-Johnson et al. 2012 [36]
‘Randomized by a permuted block algorithm’
36,282 randomized but only 2263 were asked to complete depression questionnaires at year 3 but authors did not report how they were chosen. Personal use of vitamin D supplements allowed up to 1000IU/day.
Double blind – nil else reported.
Double blind – nil else reported.
0% due to the nature of the study.While all participants were invited to complete the Burnam scale at year 1, only a subset (n=2263) were invited to complete the Burnam scale at year 3.
Yalamanchili & Gallagher 2012 [37]
Participants were randomly assigned to one of the four groups through a computer-generated randomization list.
Double blind – authors do not state method of allocation concealment.
Authors state ‘double-blind’. Participants blinded: placebo pills appeared identical to treatment pills. Authors do not state how study staff were blinded.
15%.
Sanders et al. 2011 [38]
Computer randomization of participants’ study identification number (Minitab)
An independent statistician performed allocation of treatment arm.
Double blind – participants and study staff were masked to treatment allocation until completion of study
Reported as 1% however 10% withdrew from study after randomization (these were not counted as losses to follow up).
Arasteh 1 1994 (n<100) [48]
Randomized by random number table
Scales, questionnaires and tablet vial of each individual were identified by ID numbers only.
‘Double blind’. No description.
None reported
Arasteh 2 1994 (n<100) [47]
Randomized by random number table
Scales, questionnaires and tablet vial of each individual were identified by ID numbers only.
‘Double blind’. No description.
None reported
Harris & Dawson-Hughes 1993 [53]
Women were randomly assigned after being stratified by dietary calcium intake, treatment group in previous trial, and previous category of years since menopause
‘Double blind’ – does not state how treatment allocation was concealed.
Authors state ‘double blind, placebo-controlled’ trial.
Intervention = 5.4%
Control = 4.3%
Gariballa & Forster 2007 [45]
‘Randomized’ – not stated how. Unable to obtain previous paper (2006) in which trial details are published.
All administration of treatment and assessment were done blind to treatment assignment,
Investigator and patient blinded to ongoing results of study. All patients had nutritional supplements or placebo prescribed in their drug charts, but coded as to preserve the double-blind nature of the trial.
6 weeks
Placebo group = 14%
Supplement group = 16%
6 months
Placebo group = 4%
Supplement group = 6%
Brown et al. 2001 [42]
‘Randomized’ – not state how.
A consulting statistician who was not a member of the research team designed and implemented the allocation procedures.
The investigators were purposefully vague about the number and nature of the groups. The placebo and active vitamins were identical in appearance and the bottles labeled only with a code number.
Intervention group = 5.6%
Placebo group = 9.8%
Table 5: Methodological quality of randomized control trials.
Two mixed sex trials included adults with a depressive disorder [50,51], one of which included only patients with low vitamin D levels [50]. One female-only study included those with mild-moderate depressive symptoms according to a well-validated depression scale at baseline, but who still did not have an existing clinical diagnosis of depression and who were not taking antidepressants [42]. Most other studies published baseline depressive symptom prevalences of 7% to 37% based on a cut-off on a well-validated scale.
Only two RCTs were specifically conducted among adults with low vitamin D levels [50,52]. Eight studies didn’t measure or report vitamin D levels [39,41,42,45,47-49,53]. Other baseline mean and median vitamin D levels ranged from 49nmol/L to 76.3nml/L [36- 38,44,46,50,51], which is regarded as adequate vitamin D status [54].
As with the participants, the intervention also differed widely between studies. The vitamin D3 dosage differed from low doses such as 200IU daily [41] to high doses of 5700IU daily [44]. The duration of intervention period varied from 2 years [36] to a one off injection [50]. Five RCTS used high dose vitamin D3 supplementation alone [38,43, 46,50,52] whereas three RCTs used low dose vitamin D as part of a multivitamin supplementation [41,42,45]. Six RCTs used vitamin D3 plus calcium as their intervention [36,39,44,47,48,53] and one RCT used vitamin D3 plus vitamin A [49] and one RCT used vitamin D3 plus fluoxetine [51].
Fifteen RCTs [36,37,39,41-48,50-53] measured changes in depression score before and after supplementation on validated scales. Two studies only measured depression scores after supplementation [38,49].
Methodological quality
Cohort studies: The methodological quality of the cohort studies was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) [29] and see (Table 4) for their star ratings. Milaneschi et al. 2010 [33] had the lowest risk of bias. May et al. 2010 [32] had the high risk of bias with an unclear selection protocol, no adjustment for multiple confounders (other than parathyroid hormone) and unreported loss to follow up. This study and Yue et al. 2014 [34] involved patients with a cardiovascular and cerebrovascular disease, respectively, and so have poor generalizability. Only one study used standardized interviews plus diagnostic algorithm to diagnose depression [34] whereas the rest used less reliable self-report measures [31,33,35] or a clinical diagnosis [32] to diagnose depression. Chan et al. 2011 [35] did not include those with extreme vitamin D levels, had a high loss to follow up of 21% and an inadequate assessment of outcome as they measured prevalent depression at one time point 4 years after exposure rather than a more useful outcome such as incidence over 4 years.
Randomized control trials
The methodological quality of the seventeen included trials was assessed by means of the method of randomization and allocation concealment, blinding and losses to follow-up. Please see (Table 5). Most studies used a computer generated randomization and stated that they used a ‘double blind’ procedure however not all studies explicitly described how they did this.
Other possible biases: Five studies were secondary analyses of trials looking at bone health [36-39,53]. Jorde et al. 2008 [44] was a secondary analysis of a previous study whose primary outcome was weight loss [55]. Gariballa & Forster 2007 [45] was a secondary analysis of a study whose primary outcomes were disability, length of stay and morbidity and mortality [56]. The primary outcomes for Dean et al. 2011 [46] were working memory, response inhibition & cognitive flexibility whereas the depression outcome was one of many secondary outcomes. This was similar for Wepner et al. 2014 [43] whose primary outcomes were fibromyalgia scores.
Association between vitamin D levels and depression
Cohort studies: See Supplementary (Table 1) for the hazard and odds ratios of the five studies. Four out of the five cohort studies found that older adults with ‘deficient’ or ‘insufficient’ vitamin D levels and with no known history of depression have a higher likelihood of developing depression in the following few years than older adults with ‘sufficient’ or ‘optimal’ vitamin D levels [31-34]. The overall adjusted hazard ratios ranged from 1.54 (1.19-1.99) 31 to 2.7 (1.35-5.4) p = 0.00532 to 10.32 (4.97-28.63) p<0.00134 for both men and women. Milaneschi et al. 2010 [33] stratified their results by sex and found that 130 women (43.6%) and 70 men (20.5%) developed depressed mood. After adjustment for age, baseline CES-D, ADL disabilities, use of antidepressants, number of chronic diseases, SPPB, high PTH, and season of collection, both men and women with low vitamin D levels compared to those with sufficient levels showed an increased risk of developing depression over 6 years but this was only statistically significant in women (Women: (<50nmol/l) vs. (>50nmol/l) HR=1.97(1.22-3.17) p=0.005; Men: (<50nmol/l) vs. (>50nmol/l) HR=1.61 (0.92-2.82) p=0.1;). May et al. 2010 [32] also stratified their results by sex and found large but statistically insignificant hazard ratios in males compared to females (males (<<37.5nmol/l) vs. >125nmol/L) HR 6.68, p=0.07; females (<37.5nmol/l) vs. >125nmol/L) HR 2.13, p=0.05). Both Milaneschi et al. 2010 and May et al. 2010 [33,32] did not find any statistically significant vitamin D status-sex interactions. Chan et al. 2011 [35], whose cohort consisted only of older men, did not find any statistically significant association between low vitamin D levels and increased incidence of depression.
Effects of vitamin D supplementation
Randomized control trials: Seven out of the seventeen RCTs found a statistically significant overall effect of vitamin D compared to placebo on depressive symptoms; six mixed sex trials [44,45,47,48,50,51] and one female only trial [42]. The effect sizes were as follows; -5.55 (BDI), -4.7 (HDRS) [51]; -9.3 (BDI II) [50]; -1.5 (BDI) [44]; -5.35 (BDI) [47]; -3.05 (BDI) [48]; -0.58 [45]; female only trial -8.6 (CES-D) and -24.2 (POMS) [42]. The other ten RCTs did not find any statistically significant overall effect of vitamin D compared to placebo on depressive symptoms [36-39,41,43,46,49,52,53].
Effects of vitamin D supplementation in those with depressive symptoms at baseline: Khoraminya et al. 2013 [51] and Mozaffari- Khosravi et al. 2013 [50] found a significant effect of vitamin D supplementation compared to control in improving depressive symptoms in adults with a depressive disorder. (Khoraminya et al. 2013 [51]: BDI mean change at 8 weeks: vitamin D + fluoxetine group = -17.7, fluoxetine group: -13; repeated measure analysis of variance of time and group interaction; F8.54, p=0.006. HDRS mean change at 8 weeks; vitamin D + fluoxetine group = -19.25, fluoxetine group: -13.7; repeated measure analysis of variance of time and group interaction; F6.72, p=0.013. Mozaffari-Khosravi et al. 2013 [50]: BDI-II mean change (+- SD): control group: -2.1 (+-3.8); low dose group; -6.8 (+- 7.9); high dose group: -9.3 (+-8.7), analysis of variance p<0.001.) Brown et al. 2001 [42] found a very large statistically significant effect of vitamin D supplementation in women with mild to moderate depressive symptoms (CES-D mean change: intervention group = -8.6, control group = -5.5; ANCOVA p=0.004) POMS mean change: intervention group = -24.2, control group = -18.8; ANCOVA p=0.015). In a per protocol sub analysis Jorde et al. 2008 [44] found that the improvement in BDI 1-13 in the DD (high dose vitamin D (40,000IU per week) and DP (low dose vitamin D: 20,000IU per week) groups was most clearly seen in females (Female Delta scores: DD = -2.0 (p<0.01), DP = -1.8 (p<0.05), PP (placebo) = -0.5 (p>0.05) Male delta scores: DD=-1 (p>0.05), DP= 0 (p>0.05), PP = 0 (p>0.05) who were significantly more depressed at baseline than males (Females: BDI total score: 5 (0-28), Male BDI total score: 3.5 (0-24.5) Mann- Whitney test p<0.001). Sanders et al. 2011 [38], a female only study, found a trend for those taking anxiety/antidepressant medication to score higher (i.e. better) on the SF-12 MCS if they were randomized to receive vitamin D rather than placebo, although this did not reach a statistically significant interaction (p=0.11).
Arasteh 2 1994 [47], a mixed-sex trial, split participants into ‘depressed’ and ‘non-depressed’ before randomization (‘depressed’ = baseline BDI >9, ‘non-depressed; = baseline BDI < 9). The mean BDI score of the ‘depressed’ subjects in the intervention group decreased (improved) by 11.43 points, while that of the ‘depressed’ subjects in the placebo group decreased (improved) by 4 points. This interaction between group (intervention vs. control) and mood (‘depressed’ vs. ‘non-depressed’) produced a significant effect in BDI scores (F (1,47) = 4.76, p<0.05). Kjaergaard et al. 2012 [52], a large mixed-sex trial carried out post hoc analyses by stratifying groups according to baseline depression score. Participants with higher BDI scores at baseline (using the median value 4 as a cut-off) were found to have a significant positive effect (less depressed) of vitamin D compared with placebo on total HADS score (p=0.032). Participants with high HADS and MADRS scores at baseline (using 75 percentile as cut-off) had a significant positive effect of vitamin D compared with placebo on HADS score (p=0.01 and p=0.031) respectively. In a subgroup analysis, Yalamanchili et al. 2012 [37], a female only study, found that all women who were depressed at baseline showed a significant improvement, when compared to non-depressed people at baseline, in both placebo and intervention groups (One-way ANOVA, F=68.82, p<0.0001).
Effect of vitamin D supplementation in those with low vitamin D levels: Mozaffari-Khosravi et al. 2013 [50] found a significant of vitamin D supplementation in improving depressive symptoms in adults who not only were depressed, as stated above, but also vitamin D deficient. Although Khoraminya et al. 2013 [51] did not only include those with vitamin D deficiency, 95% of their patients had insufficient vitamin D levels at baseline (<75nmol/L) and as stated above, they also found a significant effect of vitamin D supplementation on depressive symptoms. Kjaergaard et al. 2012 [52] only included participants with low serum vitamin D levels (<55nmol/L) and they did not find any significant differences in delta scores (change in BDI, HADS-D & MADRS scores before and after supplementation) between intervention and control groups, even after stratifying by baseline vitamin D level and gender. Dean et al. 2011 [46] in their secondary analysis examined the effect of change in vitamin D concentrations in only participants with low vitamin D concentrations at baseline (<75.00 nmol/L) but did not find any significant treatment effects. Jorde et al. 2008 [44] carried out a sub analysis in those with baseline vitamin D levels <40nmol/l and >40nmol/l but did not find a distinct pattern. Although four other studies [36-38,43] measured vitamin D levels at baseline and after supplementation, the majority of participants were not deficient and they did not investigate response to treatment according to baseline vitamin D levels.
Adverse Events
Randomized control trials
Ten studies reported no adverse events [36,37,39,41,45,47-50,53]. Khoraminya et al. 2013 [51] excluded one patient from the fluoxetine + vitamin D group because of severe anxiety at week 2. Wepner et al. 2014 [43] reported one case of mild hypercalcaemia (2.71 nmol/L) and a serum calcifediol level of 63.6 ng/mL in the intervention group. Sanders et al. 2011 [38] reported an increased number of serious adverse events in the supplementation group that nearly reached statistical significance (244 vitamin D vs. 207 placebo (p=0.06) but these events were not considered related to study medication. Jorde et al. 2008 [44] reported sustained hypercalcaemia in one participant in low dose vitamin D group who was removed from the trial. Brown et al. [42] found that seven participants (14%) in the control group and sixteen (30%) in the intervention groups (30%) reported a single minor side effects from the vitamins, such as bright yellow urine, gastrointestinal symptoms, sleep disturbances and skin reactions.
Discussion
Our systematic review included seventeen randomized control trials and five cohort studies. Our findings from cohort studies corroborate the association between low vitamin D levels and increased risk of depressive symptoms in both sexes, and demonstrate that female gender may indirectly strengthen this association. Milaneschi et al. 2010 [33], the most methodologically robust cohort study, found that only women, and not men, with low vitamin D levels had a significantly higher hazard of developing depressed mood during 6 years of follow up compared to women with sufficient vitamin D levels. Given that 74.6% of women and 50.4% of men in this study had vitamin D levels less than 50nmol/L at baseline; these findings are possibly secondary to the increased prevalence of vitamin D deficiency in the female sample. May et al. 2010 [32] found a larger, (but non-statistically significant) hazard ratio in men compared to women with low vitamin D levels, however this study was deemed to have a high risk of bias. Both Milaneschi et al. 2010 [33] and May et al. 2010 [32] did not find a significant interaction by sex in the association between vitamin D and depressive symptoms. Furthermore, Yue et al. 2014 [34] who found the largest increased risk of depression in men and women with severe deficiency (<28nmol/L) unfortunately did not stratify by sex. Interestingly, the only cohort study involving men only did not find any association between vitamin D levels and development of depression [35].
Regarding randomized control trials, seven out of the seventeen RCTs found that vitamin D supplementation was significantly more effective than control, in improving depressive symptoms. Six of these were mixed-sex studies and one was female only. Interestingly, the females included in these trials were mostly of childbearing age [42,44, 47,48,50,51]. Furthermore, Brown et al. 2001 [42], which showed the largest effect size of all included trials (Effect size: -8.6Regarding randomized control trials, seven out of the seventeen RCTs found that vitamin D supplementation was significantly more effective than control, in improving depressive symptoms. Six of these were mixed-sex studies and one was female only. Interestingly, the females included in these trials were mostly of childbearing age [42,44, 47,48,50,51]. Furthermore, Brown et al. 2001 [42], which showed the largest effect size of all included trials (Effect size: -8.6Regarding randomized control trials, seven out of the seventeen RCTs found that vitamin D supplementation was significantly more effective than control, in improving depressive symptoms. Six of these were mixed-sex studies and one was female only. Interestingly, the females included in these trials were mostly of childbearing age [42,44, 47,48,50,51]. Furthermore, Brown et al. 2001 [42], which showed the largest effect size of all included trials (Effect size: -8.6Regarding randomized control trials, seven out of the seventeen RCTs found that vitamin D supplementation was significantly more effective than control, in improving depressive symptoms. Six of these were mixed-sex studies and one was female only. Interestingly, the females included in these trials were mostly of childbearing age [42,44, 47,48,50,51]. Furthermore, Brown et al. 2001 [42], which showed the largest effect size of all included trials (Effect size: -8.6Regarding randomized control trials, seven out of the seventeen RCTs found that vitamin D supplementation was significantly more effective than control, in improving depressive symptoms. Six of these were mixed-sex studies and one was female only. Interestingly, the females included in these trials were mostly of childbearing age [42,44, 47,48,50,51]. Furthermore, Brown et al. 2001 [42], which showed the largest effect size of all included trials (Effect size: -8.6 using CES-D and -24.2 using POMS) was carried out purely in females (>18 years) with mild to moderate depressive symptoms. Jorde et al. 2008 [44], a mixed sex study, found their improvement in BDI 1-13 scores was most clearly seen in females, although females had significantly more depressive symptoms at baseline than men. Sanders et al. 2011 [38], a female only study, found a non-significant trend in females taking anxiety/antidepressant medication to score higher (i.e. better) on the SF-12 MCS if they were randomized to receive vitamin D rather than placebo. The two largest RCTs [36,39] that were both in postmenopausal females, did not find a significant effect of vitamin D supplementation on depressive symptoms, but this is not surprising, as the majority of these females were not depressed at baseline. Furthermore both these studies were secondary analyses and had a high degree of confounding and bias.
The other five trials that found a significant effect of vitamin D supplementation on depressive symptoms in men and women unfortunately did not stratify according to gender [45,47,48,50,51]. However the two studies with large effect sizes included 85% [51] and 72% females [50]. These two trials [50,51] were the only two trials whose participants were both depressed and vitamin D deficient, which strengthens the hypothesis that the benefit of vitamin D supplementation will be most clearly seen in those who are depressed and/or vitamin D deficient.
With regard to the biological plausibility of a link between low vitamin D levels and depressive symptoms in both sexes, there is corroborative evidence from animal and laboratory studies. Eyles et al. 2005 [19] identified vitamin D receptors in the prefrontal cortex, hippocampus, cingulated gyrus, thalamus and hypothalamus, areas of the brain that have been associated with depression. Garcion et al. 2002 [57] found that 1,25 hydroxyvitamin D3 appears to increase expression of genes encoding for tyrosine hydroxylase, the precursor of noradrenaline in the adrenal glands, a neurotransmitter implicated in depression. It is also known that vitamin D protects against serotonin-depleting effects of neurotoxic doses of methamphetamine [58]. Furthermore Feron et al. 2005 [59] found that newborn rats deprived of Vitamin D3 in utero showed micro and macro-structural brain changes that persisted into adulthood such as increased cell proliferation, larger lateral ventricles, reduced cortical thickness, reduced expression of nerve growth factor and reduced expression of glial cell line-derived neurotrophic factor. These brain changes are not specific to depression per say and are also found in schizophrenia and neurodegenerative diseases such as Alzheimer’s disease. Also, human genetic studies have shown that Vitamin D receptor polymorphisms contribute to age-related changes in depressive symptoms [60].
Women have a higher lifetime prevalence of depression [3] than men and various biological, psychological and social theories have tried to explain this difference [61]. Evidence for an underlying biological process includes the increased risk of depression in women at times of sex steroid level changes; pre-menstrually, during and after pregnancy and peri-menopausally. The female to male ratio of depression at puberty rises from 1:1 to 2:1, pointing to oestrogen and progesterone as culprits [62]. Ovarian steroids have widespread effects throughout the brain; on serotonin pathways, catecholaminergic neurons [63,64]; on the hypothalamic-pituitary-adrenal axis [65] and in fact, animal studies suggest that the amygdala, a structure involved in emotion, has one of the highest densities of oestrogen receptors in the brain [66]. Furthermore, selective serotonin uptake inhibitors are effective in treating premenstrual dysphoric disorder, a disorder with overlapping symptoms to depression [67].
Whilst obesity, old age, latitude, dark skin, sunscreen use and cultural clothing practices are clear risk factors for vitamin D deficiency, female sex as a risk factor is under dispute. Many studies have found lower levels of vitamin D in women [6-13] although there is also some evidence to the contrary [68-70]. It is thought that external factors related to sunlight exposure as well as hormonal differences underline these sex differences in circulating vitamin D levels [12,54]. However, animal and human studies show that there may be oestrogen-promoted differences in vitamin D metabolism that do not effect circulating levels of vitamin D [24,25,71], hence why vitamin D levels may not necessarily be lower in women.
Strengths and Limitations
This is the first systematic review including randomized control trial data that has investigated the gender differences in the relationship between vitamin D deficiency and depressive symptoms and in the effect of vitamin D supplementation on depressive symptoms. In view of the existence of this data we did not feel it was necessary to include further cross-sectional data given the problem of reverse causality. This review has been subject to a rigorous methodology. Studies were initially found from a systematic search of multiple databases using comprehensive search terms (see supplementary data for search terms) and were then independently assessed using pre-written inclusion criteria forms by two people. Due to the heterogeneity of the studies included in the review in terms of participants, exposures/ interventions, and outcome data, meta-analyses were not appropriate and narrative syntheses were performed. We regard this as a strength, as previous systematic reviews in this area have fallen to the temptation of meta-analysis [26, 27], despite the heterogeneity and poor quality of included studies.
There are some limitations to this systematic review that require comment. Generally, we were mostly unable to set the context in which, we hypothesized, vitamin D would have its main effects: a) in those with depressive symptoms at baseline of both sexes, b) in those who are vitamin D deficient. Out of the seventeen randomized control trials included, there were only two studies in those with clinical depression [50,51] and one other study in those with depressive symptoms at baseline [42]. Not surprisingly these three studies displayed the largest significant effect sizes. In the other studies, baseline depression score ranges revealed that a few participants had depressive symptoms indicative of a diagnosis of depression, even though baseline mean scores were low. For example in Jorde et al. 2008 [44], whilst the mean BDI score of all subjects at baseline was 5, the range was 0-28 (score >9 = mild depression, score>18=moderate depression, score>29=severe depression). Therefore the effective sample size in most studies were very small and so the power to detect a difference, low.
Secondly, whilst eight RCTs measured vitamin D levels, only two studies included only those who were vitamin D deficient at baseline [50,52], the second context in which we hypothesized vitamin D supplementation would have a main effect.
It is therefore not possible for us to make conclusive statements on whether gender modifies the effect of vitamin d supplementation and depressive symptoms, as these two contexts, in which we would expect to see a main effect of vitamin D supplementation on depressive symptoms were mostly absent. It is worth noting however that, five of the seven female-only trials [36-39,53] included postmenopausal women. Given the biological hypothesis of oestrogen promoted differences in local vitamin D metabolism, we would not expect vitamin D to have a more dramatic effect in postmenopausal women.
Conclusion
In summary, there is suggestive evidence of potential benefits of vitamin D supplementation in women of childbearing age. However, there is a need for additional large randomized placebo-controlled trials in both men and women of childbearing age, with a diagnosis of depression and vitamin D deficiency or insufficiency with genderstratified analysis to further explore this. There is no indication that vitamin D supplementation causes hypomania.
Given, the growing body of evidence that vitamin D deficiency is associated with depression as well as multiple other highly prevalent diseases, the re-introduction of regulated and targeted vitamin D fortification in food stuffs should be re-evaluated.
Acknowledgement
I would like to thank Dr Saverio Stranges, Professor Scott Weich, Dr Karen Rees, Professor Aileen Clarke and Dr Mukesh Kripalani for their contributions to this review.
References