
Research Article
Austin J Psychiatry Behav Sci. 2017; 4(1): 1059.
Psychometric Characteristics of Patient Health Questionnaire-2 (PHQ-2) in Iranian Psychiatric Outpatients
Mahboubeh Dadfar¹* and David Lester²
¹School of Behavioral Sciences and Mental Health-Tehran Institute of Psychiatry, International Campus, Iran University of Medical Sciences, Tehran, Iran
²Psychology Program, Stockton University, Galloway, NJ, USA
*Corresponding author: Mahboubeh Dadfar, School of Behavioral Sciences and Mental Health-Tehran Institute of Psychiatry, International Campus, Iran University of Medical Sciences, Tehran, Iran
Received: October 27, 2016; Accepted: February 08, 2017; Published: February 14, 2017
Abstract
Depression is a serious public health problem in community settings and primary care in the worldwide. The Patent Health Questionnaire-2 (PHQ-2) is an ultra-brief depression screener. It has been used in both research and practice settings. The PHQ-2 has not had its validity examined in psychiatric and psychological settings in Iran. A cross-sectional study was conducted to investigate reliability, validity, and factorial structure of the Farsi version of Patent Health questionnaire-2 (PHQ-2) in a convenience sample of 130 Iranian volunteer psychiatric outpatients was selected from the psychiatric and psychological clinics at the School of Behavioral Sciences and Mental Health- Tehran Institute of Psychiatry at the Iran University of Medical Sciences. They were completed the Patent Health Questionnaire-2 (PHQ-2), the Patent Health Questionnaire-9 (PHQ-9), the Patent Health Questionnaire-15 (PHQ-15), the World Health Organization-five Well-Being Index (WHO-5), and the short form of the Beck Depression Inventory-13 (BDI-13). The mean score of the PHQ-2 was 3.53 (SD=1.73). The Cronbach alpha, Spearman-Brown, Guttman Split-Half coefficients, and one-week test-retest reliability for the PHQ-2 were 0.74, 0.74, 0.74, and 0.76, respectively. The PHQ-2 correlated 0.80 with the PHQ-9, 0.45 with the PHQ-15, -0.45 with the WHO-5, and 0.69 with the BDI-13, indicating good construct and criterion-related validity. The results of the factor analysis of the PHQ-2 items identified 1 factor labeled: General depression (79.44% of the variance). The PHQ-2, therefore, appears to have a uni dimensional structure, acceptable validity and reliability, and it can be used in the primary care, general population, clinical, and research settings in Iran society.
Keywords: Depression; Validity; Reliability; Factorial structure; Patent health questionnaire-2; Psychiatric outpatients; Iran
Introduction
Mental disorders are a major contributor to the Years Living with Disability (YLD) in worldwide [1]. Depression is a serious public health problem, the most prevalent and treatable mental disorder in primary care [2]; and in community settings [3]. The Patent Health Questionnaire-2 (PHQ-2) is a measure for diagnosing and monitoring depression [4-6]. It is a brief multipurpose, useful and time-saving measure for detecting, severity, and monitoring outcomes of depression over time [7-9].
The goal of the PHQ-2 is to screen for depression in a “first step” approach. Patients who screen positive should be further evaluated with the Patent Health Questionnaire-9 (PHQ-9) to determine whether they meet criteria for a depressive disorder. Clinical utility of the PHQ-2 is to reduce depression evaluation to two screening questions relating to core symptoms of depression (i.e. low mood, and loss of interest or pleasure) enhance routine inquiry about this mental disorder [8,10-12].
Lino, Portela, Camacho, Atie, Lima, et al. [13] reported that the PHQ-2 is not sufficient to screen for depression, and it is the first step of the screening. Mitchell, Yadegarfar, Gill, and Stubbs [14] indicated that although the PHQ-2 has sensitivity and specificity, and is adequate for initial first step assessment of depression in primary care, but it cannot confirm a clinical diagnosis. Nevertheless, using of very shorter and brief screening tools for diagnosis and management of depression has been interested in many settings [15].
The PHQ-2 has not had its validity examined in psychiatric and psychological settings in Iran. We examined psychometric and screening properties for depression of this questionnaire in a sample of Iranian psychiatric outpatients.
Methods
Participants
A convenience sample of 130 Iranian volunteer psychiatric outpatients was selected from the psychiatric and psychological clinics at the School of Behavioral Sciences and Mental Health- Tehran Institute of Psychiatry at the Iran University of Medical Sciences in Iran. The mean age of the patients was 31.40 years (SD= 8.20); the mean duration of their mental disorder was 7.91 years (SD=6.94); 73.4% were female; 62.4% were single; 29.6% were married, 6.4% divorced, and .8% widow; the majority 66.6% had a degree of between lower diploma and higher diploma, 33.4% between BA, to Ph.D. degree; 55.6% had an anxiety disorder, 37% a depressive disorder, 3.% other disorders, and 7.3% missing data). They were completed the Farsi versions of the Patent Health Questionnaire-2 (PHQ-2), the Patent Health Questionnaire-9 (PHQ-9), the Patent Health Questionnaire-15 (PHQ-15), the World Health Organizationfive Well-Being Index (WHO-5), and the short form of the Beck Depression Inventory-13 (BDI-13).
Measures
The Patent Health Questionnaire-2 (PHQ-2): The PHQ-2 inquires about the frequency of depressed mood and anhedonia over the past two weeks. It includes the first two items of the PHQ-9. Each item is scored four-point Likert scale from 0 to 3. The PHQ-2 total score for the two items ranges from 0 to 6: Not at all (0), Several days (1), More than half the days (2), and Nearly every day (3). For major depressive disorder (7% prevalence) the sensitivity, specificity, and Positive Predictive Value (PPV) of the PHQ-2 were 97.6, 59.2, and 15.4 (for score of 1); 92.7, 73.7, and 21.1 (for score of 2); 82.9, 90.0, and 38.4 (for score of 3); 73.2, and 93.3, 45.5 (for score of 4); 53.7, 96.8, and 56.4 (for score of 5); 26.8, 99.4, and 78.6 (for score of 6), respectively. For any depressive disorder (18% prevalence) the sensitivity, specificity, and PPV of the PHQ-2 were 90.6, 65.4, and 36.9 (for score of 1); 82.1, 80.4, and 48.3 (for score of 2); 62.3, 95.4, and 75.0 (for score of 3); 50.9, and 97.9, 81.2 (for score of 4); 31.1, 98.7, and 84.6 (for score of 5); 12.3, 99.8, and 92.9 (for score of 6), respectively [4,8,10]. Kroenke, et al. [4] identified a PHQ-2 cutoff score of 3 as the optimal cut point for screening purposes and stated that a cut point of 2 would enhance sensitivity, whereas a cut point of 4 would improve specificity. In study of Löwe, et al. [7], with reference to the Structured Clinical Interview for DSM-IV (SCID), the PHQ-2 had a sensitivity of 87% and a specificity of 78% for major depressive disorder and a sensitivity of 79% and a specificity of 86% for any depressive disorder. McManus, Pipkin, and Whooley [16], with reference to the Diagnostic Interview Schedule, showed that the PHQ-2 had a sensitivity of 39% and a specificity of 92% for screening depression in patients with Coronary Heart Disease (CHD). Li, Friedman, Conwell, and Fiscella [17], with reference to the Structured Clinical Interview for DSM-IV (SCID), reported that the PHQ-2 had a sensitivity of 100%, a specificity of 77%, and Area Under the receiver operating characteristic Curve (AUC) 0.88 for identifying major depression in older patients. Cutler, Legano, Dreyer, Fierman, Berkule, et al. (2007), with reference to the Edinburgh postnatal depression scale, found that that the PHQ-2 had a sensitivity of 43% and a specificity of 97% for screening of maternal depression in a low-education population. Lima Osório, Vilela Mendes, Crippa, & Loureiro [18], with reference to the SCID, indicated the best cutoff score for the Brazilian version of the PHQ-2 was between 3 and 4. Richardson, et al [10] found the PHQ-2 score of > or =3 had a sensitivity of 74% and specificity of 75% for detecting major depression in adolescent in primary care, also it had an area under the curve of 0.84. Arroll et al [8] validated the PHQ-2 for diagnosing major depression, with reference to the computerized Composite International Diagnostic Interview (CIDI), and indicated that sensitivity and specificity of the PHQ-2 were 86% and 78% with a score of 2 or higher; and 61% and 92% with a score 3 or higher, respectively. On the PHQ-2, a score of 2 or higher detected more cases of depression than a score of 3 or higher in the primary care population of New Zealand. Similar to these findings, Lino et al. [13], with reference to the SCID, found that the PHQ-2 had sensitivity 0.74, specificity 0.77, PPV 0.50, Negative Predictive Value (NPV) 0.90, with score equal to 1, and the AUC was 0.77. Hanwella, Ekanayake, and de Silva [19], with reference to the SCID-II, showed the sensitivity and the specificity of the PHQ-2 were 0.80, and 0.97, respectively. Manea et al. [15] using a systematic review found that sensitivity and specificity of the PHQ-2 were 91%, and 70% with a score 2 or higher; and 76% and 87% with a score of 3 or higher, respectively. On the PHQ-2, a score of 3 or higher had lower sensitivity than 83% in the original validation study (with a score of 2 or higher), donating a score of 2 or higher is preferable in identifying depression. In study of Liu, Yu, Hu, Lin, Zhou, et al. [20], the Cronbach’s alphas of PHQ-2 was 0.76. With score of 3 of PHQ-2, the highest Youden’s index of 0.79, with both sensitivity and specificity were 0.90 and the AUC was 0.94 to screening depression in the Chinese rural elderly. They suggested cut-off score of 3 for the PHQ-2.
The Patent Health Questionnaire-9 (PHQ-9): The PHQ-9 is a self-administered popular scale for assessing, diagnosing, and monitoring of depression severity, is sometimes used in certain screening or research settings [21-25]. The PHQ-9 has nine items and the answers refer to the past two weeks. Each item is scored fourpoint Likert scale from 0 to 3. The total score for the nine items ranges from 0 to 27: Not at all (0), Several days (1), More than half the days (2), and Nearly every day (3). Severity of depression is scored noneminimal (0-4); mild (5-9); moderate (10-14); moderately severe (15- 19); and sever (20-27) [27-29]. Psychometric properties of the PHQ- 9 have been investigated in many studies and good reliability and validity have been reported for the scale [17,19,26,27,30-45].
The Patent Health Questionnaire-15 (PHQ-15): The PHQ-15 is a brief, self-administered measure for screening of somato form disorders e.g. somatization, evaluating and monitoring the severity of somatic symptoms in clinical practice and research settings. It comprises 15 somatic symptoms (During the past four weeks, how much have you been bothered by any of the following problems: stomach pain, back pain, pain in your arms or legs or other joints, menstrual cramps or other problems with your periods (women only), headaches, chest pain, dizziness, fainting spells, feeling your heart pound or race, shortness of breath, pain or problems during sexual intercourse, constipation, loose bowels, or diarrhea, nausea, gas, or indigestion, feeling tired, or having low energy, and trouble sleeping) from the PHQ of the PRIME-MD, each symptom scored in Not bothered at all (0), Bothered a little (1), and Bothered a lot (2). The PHQ-15 scores of 5, 10, 15, represented cutoff points for low, medium, and high somatic symptom severity, respectively. Scores ranged to 0-4 (no somatisation disorder), 5-9 (mild somatisation disorder), 10-14 (moderate somatisation disorder), and 15+ (severe somatisation disorder) [46]. Evidence indicates high reliability and validity of the PHQ-15 in different samples of various settings [47- 54].
The World Health Organization-five Well-Being Index (WHO-5): The 5-item World Health Organization well-being index was developed at the Psychiatric Research Unit, Mental Health Centre North Zealand, Hillerod, Denmark. The WHO-5 is a commonly used measure of subjective psychological well-being, and emotional well-being, and is a screening tool for depression and as an outcome instrument in clinical trials [55]. Each of the five items of the WHQ-5 is rated on a 6-point Likert scale from not present (0) to constantly present (5). The lower the total score is, the more severe the depression, poor physical health, and psychological health, the higher the total score, the better the physical and psychological health. An answered score of 1 or 0 on any of these items means that it may be helpful to consult with a counseling professional. A score of 13 or lower suggests further investigation into possible symptoms of depression. It is suggested to administer the major depression (ICD-10) inventory if the raw score is below 13 or if the patient has answered 0 to 1 to any of the five items. Scores are summated, with a raw score ranging from 0 to 25, and the total score is multiplied by 4 in order to obtain a percentage score, with higher scores meaning better well-being. A percentage score of 0 represents the worst possible well-being, while a score of 100 represents the best possible well-being. A score of 50 or below is indicative of low mood, though not necessarily depression, and a score of 28 or below indicates likely depression and warrants further assessment (diagnostic interview) to confirm depression [32]. Acceptable psychometric characteristics of the WHO-5 have been shown in previous studies in different samples, e.g. for depression in Dutch diabetes outpatients [56,57] in primary care patients [58]; for screening of psychological wellbeing in patients with Metabolic Syndrome (MS) [28]; for screening of well-being in Iranian adolescents [59]; and for maternal wellbeing in Iranian pregnant women [60]. The test-retest reliability of the WHO-5 in Germany and Japan was 0.90. Wu [28] indicated the WHO-5 negatively correlated -0.60 with the PHQ-9, -0.42 with the Hospital Anxiety and Depression scale (HADS-Anxiety), -0.57 with the HADS-Depression, and positively correlated 0.49 with the World Health Organization Quality of Life-Short-form Version for Taiwan (WHOQOL) in patients with metabolic syndrome [28].
The short form of the Beck Depression Inventory-13 (BDI-13): The BDI-13 is a screening tool for depressive disorders. Dadfar and Kalibatseva [61] found good psychometric properties for the BDI-13 with Iranian psychiatric outpatients including Cronbach’s alpha of .85, having moderate to strong positive associations of the BDI-13 with the instruments related to mental health constructs, and three identified factors were affective, somatic/vegetative, and cognitive/ loss of functioning.
Results
The mean score of the PHQ-2 was 3.53 (SD=1.73). The lowest mean score was 1.66 (SD=.96) for item of 1, and the highest mean score was 1.87 (SD=.97) for item of 2 (Table 1).
PHQ-9
Minimum
Maximum
Mean
SD
1
0
3
1.66
0.96
2
0
3
1.87
0.97
Total score
0
6
3.53
1.73
Table 1: Mean and SD of the PHQ-2 items and total score.
Reliability coefficients of the PHQ-2
The Cronbach alpha coefficient for the PHQ-2 was 0.74 the Spearman-Brown coefficient 0.74, the Guttman Split-Half coefficient 0.74, indicating high internal consistency (Table 2). One-week testretest reliability was 0.76.
Questionnaires
Mean
SD
Number of items
Format
Cronbach’s Alpha
Patient Health Questionnaire-2 (PHQ-2)
3.53
1.73
2
Likert (0-3)
0.74
Patient Health Questionnaire-9 (PHQ-9)
12.83
6.25
9
Likert (0-3)
0.88
Patient Health Questionnaire-15 (PHQ-15)
11.02
6.22
15
Likert (0-2)
0.85
World Health Organization-five Well-Being Index (WHO-5)
9.05
6.46
5
Likert (0-6)
0.92
Beck Depression Inventory-13 (BDI-13)
9.65
5.92
13
Likert (0-3)
0.8
Table 2: Descriptive statistics of all questionnaires.
Correlations of inter-items, and total scores of the PHQ-2
The correlations between items and total score were ranged from .892 for item of 2 and total score to .891 for item of 1 and total score (significant at the 0.01 level), indicating high association between the each items and total scores of the scale.
The correlations between items were ranged .589 for items of 1 and 2 (significant at the 0.01 level), indicating moderate association between the each items of the scale (Table 3).
Items
1
2
Total
1
1
2
.589**
1
Total
.891**
.892**
1
**Significant at the 0.01 level.
Table 3: The Pearson correlations (r) between the PHQ-2 items and total.
Correlations of the PHQ-2 with other questionnaires
The PHQ-2 correlated 0.80 with the PHQ-9, 0.45 with the PHQ- 15, -0.45 with the WHO-5, and 0.69 with the BDI-13, indicating moderate to high construct and criterion-related validity and association between the measures. Concurrent validity for the PHQ- 2 with the other scales, were positively significant at the 0.01 level, expect for PHQ-2 with WHO-5 was negatively significant at the 0.01 level (Table 4).
Questionnaires
r with PHQ-2
Patient Health Questionnaire-9 (PHQ-9)
.800**
Patient Health Questionnaire-15 (PHQ-15)
.458**
World Health Organization-five Well-Being Index (WHO-5)
-.457**
Beck Depression Inventory-13 (BDI-13)
.698**
**Significant at the 0.01 level.
Table 4: The Pearson correlations (r) between the questionnaires.
Factor analysis of the PHQ-2
The criteria for the factor analysis were evaluated using the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO) and the Bartlett Test of Sphericity. The KMO was 0.500, indicating the adequacy of the sample of psychiatric outpatients, and the Bartlett’s Test of Sphericity was 54.286 (df = 1, p< .001) indicating that the factor analysis was justified in the psychiatric outpatients sample. The results of exploratory factor analysis on PHQ-2 extracted only one component (factor), and the solution cannot be rotated.
Factor 1 (2 items) explained 79.44% of the observed variance and was labeled “General depression”. It included the item: “There have been times when I wished that I were dead”, and “I sometimes think that death would solve my problems” (Table 5 and Figure 1).
Patient Health Questionnaire-2 Items
Over the last 2 weeks‚ how often have you been bothered by any of the following problems?
Component
1
1. Little interest or pleasure in doing things.
.89
2. Feeling down‚ depressed‚ or hopeless.
.89
Eigen value
% of Variance
1.58
79.44
Factor 1 (item: 1 and 2): General depression.
Table 5: Factor loadings of the Patient Health Questionnaire-2 in 130 Iranian psychiatric outpatients.
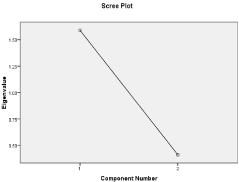
Figure 1: Screen Plot for the PHQ-2.
Conclusion
The aim of the study was to examine of psychometric and screening properties for depression of the PHQ-2 in a sample of Iranian psychiatric outpatients.
The mean score of the PHQ-2 was 3.53 (SD=1.73). The lowest mean score was 1.66 (SD=.96) for item of 1 “Little interest or pleasure in doing things”, and the highest mean score was 1.87 (SD=.97) for item of 2 “Feeling down,depressed,or hopeless”.
We found the Cronbach alpha, Spearman-Brown, Guttman Split- Half coefficients, and one-week test-retest reliability for the PHQ-2 was 0.74, 0.74, 0.74, and 0.76, respectively, indicating good reliability. Similar to our finding, the study of Liu et al [20] showed that the PHQ-2 has good Cronbach’s alpha 0.76.
Our study showed that the correlations between two items; and between two items and total score were significant at the 0.01 level. Liu et al [20] found correlations between the total scores of the PHQ- 2 and each item were 0.81 and 0.90, respectively.
The PHQ-2 correlated 0.80 with the PHQ-9, 0.45 with the PHQ- 15, -0.45 with the WHO-5, and 0.69 with the BDI-13, positively significant at the .01 level (expect for PHQ-2 with WHO-5 was negatively significant at the 0.01 level) and indicating good construct and criterion-related validity. Findings of Li et al [17] showed the PHQ-2 had adequate criterion validity and correlated with the six scales of the Medical Outcomes Study 12-item Short Form Questionnaire (SF-12), donating good construct validity. Lima Osório, et al [18] identified discriminative validity of the PHQ-2 and the PHQ-9.
We identified 1 factor labeled: General depression (79.44% 0f the variance). The validation process of the Farsi PHQ-2 version showed psychometric properties similar to those in international studies, indicating the PHQ-2 assesses the same constructs, in the same way, as the original version. We provided evidence for the validity and reliability of the PHQ-2 as a quick screening instrument, or a brief tool of depression severity in Iranian patients with psychiatric disorders. The PHQ-2, therefore, appears to have a uni dimensional structure, adequate and good validity and reliability, and it can be can be administered easily and used in the primary care, general population, clinical, and research settings in Iran society.
For evaluation the diagnostic accuracy of minor and major depression by the PHQ-2, combination the use of an interviewbased ICD (International Classification of Diseases) or DSM (Diagnostic and statistical Manual of Mental Disorders) diagnosis of depression, the Mini International Neuropsychiatric Interview (MINI), a semi structured interview, the Computerized Diagnostic Interview Schedule (C-DIS), the Composite International Diagnostic Interview (CIDI), the Center for Epidemiological Studies Depression (CES-D) Scale, the PHQ-9, the 90-item Revised Symptoms Checklist (SCL-90-R), the 28-item General Health Questionnaire (GHQ-28), the 10-item/6-item Kessler Psychological Distress Scales (K10/K6), the Patient Health Questionnaire–Somatic, Anxiety, Depressive Symptoms (PHQ-SADS), and using Rasch Item Response Theory (IRT) approaches are recommended.
References
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study. The Lancet. 2012; 380: 2163-2196.
- Siu US. Preventive Services Task Force (USPSTF). Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. The Journal of the American Medical Association (JAMA). 2016; 315: 380-387.
- Mojtabai R. Clinician-identified depression in community settings: concordance with structured-interview diagnoses. Psychotherapy and Psychosomatic. 2013; 82: 161-169.
- Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: validity of a two-item depression screener. Medical Care. 2003; 41: 1284-1292.
- Kroenke K, Spitzer RL, Williams JBW, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. General Hospital Psychiatry. 2010; 32: 345-359.
- Loeb D, Sieja A, Corral J, Zehnder NG, Guiton G, Nease DE. Evaluation of the role of training in the implementation of a depression screening and treatment protocol in 2 academic outpatient internal medicine clinics utilizing the electronic medical record. American Journal of Medical Quality (AJMQ). 2015; 30: 359-366.
- Löwe B, Kroenke K, Grafe K. Detecting and monitoring depression with a two-item questionnaire (PHQ-2). Journal of Psychosomatic Research. 2005; 58: 163-171.
- Arroll B, Goodyear-Smith F, Crengle S, Gunn J, Kerse N, Fishman T, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Annals of Family Medicine. 2010; 4: 348-353.
- Maurer DM, Do MPH, Darnall CR. Screening for depression. American Family Physician. 2012; 85: 139-144.
- Richardson LP, Rockhill C, Russo JE, Grossman DC, Richards J, McCarty C, et al. Evaluation of the PHQ-2 as a brief screen for detecting major depression among adolescents. Pediatrics. 2010; 125: 1097–1103.
- Thombs BD, Benedetti A, Kloda LA, Levis B, Nicolau I, Cuijpers P, et al. The diagnostic accuracy of the Patient Health Questionnaire-2 (PHQ-2), Patient Health Questionnaire-8 (PHQ-8), and Patient Health Questionnaire-9 (PHQ-9) for detecting major depression: protocol for a systematic review and individual patient data meta-analyses. Systematic Review. 2014.
- Beamish C, Damman L. Use of PHQ-2 and PHQ-9 Patient Health Questionnaires for depression screening in primary care. Behavioral Health Project, Fairview Health Services. 2016.
- Lino VT, Portela MC, Camacho LA, Atie S, Lima MJ, Rodrigues NC, et al. Screening for depression in low-income elderly patients at the primary care: use of the patient health questionnaire-2. PLoS One. 2014; 9: 12.
- Mitchell AJ, Yadegarfar M, Gill J, Stubbs B. Case finding and screening clinical utility of the Patient Health Questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. British Journal of Psychiatry Open. 2016; 2: 127-138.
- Manea L, Gilbody S, Hewitt C, North A, Plummer F, Richardson R, et al. Identifying depression with the PHQ-2: A diagnostic meta-analysis. Journal of Affective Disorders. 2016; 203: 382-395.
- McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study). American Journal of Cardiology. 2005; 96: 1076-1081.
- Li C, Friedman B, Conwell Y, Fiscella K. Validity of the Patient Health Questionnaire 2 (PHQ-2) in identifying major depression in older people. Journal of the American Geriatrics Society. 2007; 55: 596-602.
- Lima Osorio F, Vilela Mendes A, Crippa JA, Loureiro SR. Study of the discriminative validity of the PHQ-9 and PHQ-2 in a sample of Brazilian women in the context of primary health care. Perspectives in Psychiatric Care. 2009; 45: 216-227.
- Hanwella R, Ekanayake S, De Silva VA. The validity and reliability of the Sinhala translation of the Patient Health Questionnaire (PHQ-9) and PHQ-2 screener. Depression Research and Treatment. 2014.
- Liu ZW, Yu Y, Hu M, Lin HM, Zhou L, Xiao SY. PHQ-9 and PHQ-2 for screening depression in Chinese rural elderly. PLoS One. 2016; 11: e0151042.
- Johnson JG, Harris ES, Spitzer RL, Williams JBW. The Patient Health Questionnaire for Adolescents: Validation of an instrument for the assessment of mental disorders among adolescent primary care patients. Journal of Adolescent Health. 2013; 30: 196-204.
- Kroenke K, Spitzer RL, Williams JBW, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009; 50: 613-621.
- Khamseh ME, Baradaran HR, Javanbakht A, Mirghorbani M, Yadollahi Z, Malek M. Comparison of the CES-D and PHQ-9 depression scales in people with type 2 diabetes in Tehran, Iran. BMC Psychiatry. 2011; 11: 61.
- Carlota R, Sales C, Elliott R. Why using individualized outcome measures in mental health? A thematic comparison of patient-generated items in PQ with CORE-OM and PHQ-9. Master of thesis in psychology specialization in health and clinical psychology, department of psychology, Social Science School, University of Evora, Portugal. 2006.
- Neves I, Sales C, Carlota R, Brinquete C, Magalhes A, Falco C, et al. Relevance of indiviualized measures: Listening the patient means capturing the story? Psicologia, Saúde & Doenças. 2016; 17: 39-44.
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric Annals. 2002; 32: 509-521.
- Löwe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Medical Care. 2004; 42: 1194-1201.
- Wu SFV. Rapid screening of psychological well-being of patients with chronic illness: Reliability and validity test on WHO-5 and PHQ-9 scales. Depression Research and Treatment. 2014.
- Torres A, Monteiro S, Pereira A, Albuquerque E. Reliability and validity of the PHQ-9 in Portuguese women with breast cancer. The European proceeding of Social & Behavioral sciences EPSBS Future Academy Publication. And paper presented at 2nd International Conference on Health and Health Psychology icH & Hpsy. 2016.
- Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): A diagnostic meta-analysis. Journal of General Internal Medicine. 2007; 22: 1596-1602.
- Carballeira Y, Dumont P, Borgacci S, Rentsch D, Tonnac N, Archinard M, et al. Criterion validity of the French version of Patient Health Questionnaire (PHQ) in a hospital department of internal medicine. Psychology and Psychotherapy: Theory, Research and Practice. 2007; 80: 69-77.
- Yeung A, Fung F, Yu S, Vorono S, Ly M, Wu S. Validation of the Patient Health Questionnaire-9 for depression screening among Chinese Americans. Comprehensive Psychiatry. 2008; 49: 211-217.
- Gjerdingen D, Crow S, McGovern P, Miner M, Center B. Postpartum depression screening at well child visits: validity of a 2-question screen and the PHQ-9. Annals of Family Medicine. 2009; 7: 63-70.
- Osório FL, Mendes AV, Crippa JA, Loureiro SR. Study of the discriminative validity of the PHQ-9 and PHQ-2 in a sample of Brazilian women in the context of primary health care. Perspectives in Psychiatric Care. 2009; 45: 216-227.
- Zuithoff NP, Vergouwe Y, King M, Nazareth I., Van Wezep MJ, Moons KG, et al. The Patient Health Questionnaire-9 for detection of major depressive disorder in primary care: consequences of current thresholds in a cross sectional study. BMC Family Practice. 2010; 11: 98.
- Hyphantis T, Kotsis K, Voulgari PV, Tsifetaki N, Creed F, Drosos AA. Diagnostic accuracy, internal consistency, and convergent validity of the Greek version of the patient health questionnaire 9 in diagnosing depression in rheumatologic disorders. Arthritis Care & Research (Hoboken). 2011; 63: 1313-1321.
- Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. Canadian Medical Association Journal. 2002; 184: 191-196.
- Inagaki M, Ohtsuki T, Yonemoto N, Kawashima Y, Saitoh A, Oikawa Y, et al. Validity of the Patient Health Questionnaire (PHQ)-9 and PHQ-2 in general internal medicine primary care at a Japanese rural hospital: a cross-sectional study. General Hospital Psychiatry: Psychiatry, Medicine and Primary Care. 2013; 35: 592-597.
- Santos I, Tavares BF, Munhoz TN, Almeida LSPD, Silva NTBD, Tams BD, et al. Sensitivity and specificity of the Patient Health Questionnaire-9 (PHQ-9) among adults from the general population. Cadernos de Saúde Pública Rio de Janeiro. 2013; 29: 1533–1543.
- Rathore JS, Jehi LE, Fan Y, Patel SI, Foldvary-Schaefer N, Ramirez MJ, et al. Validation of the Patient Health Questionnaire-9 (PHQ-9) for depression screening in adults with epilepsy. Epilepsy & Behavior. 2014; 37: 215-220.
- Zhong Q, Gelaye B, Fann JR, Sanchez SE, Williams MA. Cross-cultural validity of the Spanish version of PHQ-9 among pregnant Peruvian women: a Rasch item response theory analysis. Journal of Affective Disorders. 2014; 158: 148-153.
- Zhong Q, Gelaye B, Rondon M, Sanchez SE, Garcia PJ, Sanchez E, et al. Comparative performance of Patient Health Questionnaire-9 and Edinburgh Postnatal Depression Scale for screening antepartum depression. Journal of Affective Disorders. 2014; 162: 1-7.
- Xiong N, Fritzsche K, Wei J, Hong X, Leonhart R, Zhao X, et al. Validation of patient health questionnaire (PHQ) for major depression in Chinese outpatients with multiple somatic symptoms: a multicenter cross-sectional study. Journal of Affective Disorders. 2015; 174: 636-643.
- Solem S, Thunes SS, Hjemdal O, Hagen R, Wells A. A metacognitive perspective on mindfulness: An empirical investigation. BMC Psychology. 2015; 3: 24.
- Van der Zwaam GL, Van Dijk SE, Adriaanse MC, Van Marwijk HW, Van Tulder MW, Pols AD, et al. Diagnostic accuracy of the patient health questionnaire-9 for assessment of depression in type II diabetes mellitus and/or coronary heart disease in primary care. Journal of Affective Disorders. 2016; 15: 68-74.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic Medicine. 2002; 64: 258-266.
- Han C, Pae CU, Patkar AA, Masand PS, Kim KW, Joe SH, et al. Psychometric properties of the Patient Health Questionnaire-15 (PHQ-15) for measuring the somatic symptoms of psychiatric outpatients. Psychosomatics. 2009; 50: 580-585.
- Ros Montalban S, Comas Vives A, Garcia-Garcia M. Validation of the Spanish Version of the PHQ-15 Questionnaire for the evaluation of physical symptoms in patients with depression and/or anxiety disorders: DEPRE-SOMA study. Actas Espan~olas de Psiquiatria. 2010; 38: 345-357.
- Lee S, Ma YL, Tsang A. Psychometric properties of the Chinese 15-item Patient Health Questionnaire in the general population of Hong Kong. Journal of Psychosomatic Research. 2011; 71: 69–73.
- De Vroege L,
- R, Nuyen J, Sijtsma K, Van der Feltz- Cornelis CM. Validation of the PHQ-15 for somatoform disorder in the occupational health care setting. Journal of Occupational Rehabilitation. 2012; 22: 51-58.
- Kocalevent RD, Hinz A, Brahler E. Standardization of a screening instrument (PHQ-15) for somatization syndromes in the general population. BMC Psychiatry. 2013; 13: 91.
- Qian J, Ren ZQ, Yu DH, He XY, Li CB. The value of the Patient Health Questionnaire-15 (PHQ-15) for screening somatic symptoms in general hospital. Chinese Mental Health Journal. 2014; 28: 173–178.
- Gierk B, Kohlmann S, Toussaint A, Waha I, Brunahl CA, Murray AM. Assessing somatic symptom burden: A psychometric comparison of the Patient Health Questionnaire-15 (PHQ-15) and the Somatic Symptom Scale-8 (SSS-8). Journal of Psychosomatic Research. 2015; 78: 352–355.
- Zhong L, Fritzsche K, Liu Y, Wang J, Huang M, Wang Y, et al. Validation of the Chinese version of the PHQ-15 in a tertiary hospital. BMC Psychiatry. 2016; 16: 89.
- Topp CW, Ostergaard SD, SØndergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychotherapy and Psychosomatics. 2015; 84: 167-176.
- De Wit M, Pouwer F, Gemke RJBJ, Delemarre-van de Waal, Snoek FJ. Validation of the WHO-5 well-being index in adolescents with type 1 diabetes. Diabetes Care. 2007; 30: 2003–2006.
- Haios TR, Pouwer F, Skovlund SE, Den Oudsten BL, Geelhoed-Dujvestijn PH, Tack CL, et al. Psychometric and screening properties of the WHO-5 well-being index in adult outpatients with Type 1 or Type 2 diabetes mellitus. Diabetic Medicine. 2013; 30: 63-69.
- Saipanish R, Lotrakul M, Sumrithe S. Reliability and validity of the Thai version of the WHO-Five Well-Being Index in primary care patients. Psychiatry and Clinical Neurosciences. 2009; 63: 141-146.
- Khosravi A, Mousavi SA, Chaman R, Sepidar Kish M, Ashrafi E, Khalili M, et al. Reliability and validity of the Persian version of the world health organization-five well-being index. International Journal of Health Studies. 2017; 1: 17-19.
- Mortazavi F, Mousavi SA, Chaman R, Khosravi A. Validation of the World Health Organization-5 Well-Being Index; assessment of maternal well-being and its associated factors. Türkiye Psikiyatri Derneği (TPD). 2015; 1: 48-55.