
Case Series
Austin J Radiol. 2015;2(1): 1011.
Atretic Meningocele: Etiopathogenesis, Frequency, Anomaly Associations and Imaging Findings
Suheil Artul1,2*, William Nseir2,3 Faozi Artoul4, Bishara Bisharat2,3and George Habib3
1Department of Radiology, Nazareth Hospital, Israel
2Faculty Medicine in the Galilee, Bar-Ilan University, Israel
3Department of Medicine, Nazareth Hospital, Israel
4Department of Medicine, Meir Hospital, Israel
*Corresponding author: Suheil Artul, Department of Radiology, Nazareth Hospital, EMMS, Bar Ilan university, faculty of medicine, Israel
Received: January 22, 2015; Accepted: February 22, 2015; Published: February 24, 2015
Abstract
Atretic meningocele is a rare subtype of encefalocele. It represents a small, skin-covered, sub scalp lesion that contains meanings, neural and glial rests.
Encephalocele in general is a very rare congenital malformation of the central nervous system. It is the result of failure of the surface ectoderm to separate from the neuroectoderm. This leads to a bony defect which allows herniation of the meanings or brain tissue and spinal fluids.
Atretic meningocele is usually located in the midline of the parietal or occipital region.
In this paper we will try to deal with the etiopathogenesis, frequency, associated anomalies and with imaging findings.
Keywords: Atretic meningocele; Etiopathogenesis; Frequency; Anomaly associations and imaging findings
Introduction
In 1964, McLaurin reported seven children with a diagnosis of parietal meningocele, among a series of 13 children with parietal cephaloceles. Since that time, a number of investigators had reported small, skin-covered midline sub scalp masses or cysts variably described as atretic cephaloceles, atypical meningoceles, rudimentary meningoceles, meningeal heterotopias, or meningocele manque. These rare lesions generally occur within a few centimeters of the lambda, which in adults is the occipital rounded angle that corresponds to the point of meeting of the sagittal suture with the lamboide suture (Figure 1). In fetal life it corresponds to the posterior fontanel. These lesions contain meninges and neural rests, with approximately half of the reported cases are parietal in location.
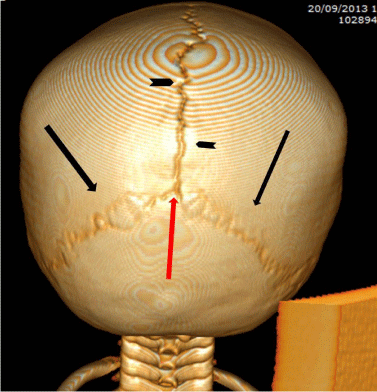
Figure 1: 3D CT reconstruction posterior view of head, show the lambda (red arrow) which is the point of meeting of the sagittal suture (black arrowheads) with the lamboide sutures (black arrows).
Atretic encephaloceles are frequently solid lesions due to hamartomatous proliferation of fibrous tissue and blood vessels. Due to the fibrous nature of the tumor (with no cystic cavity and unusual location with no connection to Central Nervous System (CNS) they are frequently regarded as insignificant hamartomas. Apart from this terminology, they are also described as cutaneous meningiomas or hamartomas with ectopic meningothelial elements for the presence of meningothelial cells. The term atretic encephalocele is more appropriate for these cases because it alludes to the pathogenesis and the non-neoplastic nature of the lesion [1-3].
Atretic cephaloceles may be related with a number of anomalies including corpus callosum agenesis, dermal sinus, hydrocephalus, Chiari II malformation, Dandy-Walker malformation and macrocephaly [2-4].
Discussion
Cephaloceles is a congenital herniation of intracranial contents through a defect in the skull. It is considered a type of Neural Tube Defect (NTD). If the herniated portion contains meanings or cerebrospinal fluid, it is termed a meningocele, whereas an encephalocele contains any amount of brain tissue, as well as meninges or cerebrospinal fluid. Cephaloceles most often occur along the midline of the skull, with most being located over the occiputum. The term atretic meningocele denotes a small, noncystic, flat or nodular lesion, in contrast with the larger pedunculated masses also seen. The atretic form represents about half of all such lesions [4,5] (Figure 2).
Figure 2a: Doppler US of right frontal bone show 1 cm hypo echoic mass (circle) without flow and show the defect in the internal part of diplopic of bone (arrow).
Figure 2b: Axial CT of head (bone window) show the same lytic frontal lesion (circle) with internal bone defect (arrow) was done exision of this unusual frontal presentation of the lesion and histology revealed total osseous atretic meningocele with remnants of meningothelial elements.
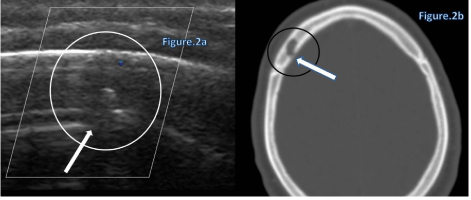
Figure 2: 22 –years old man referred to ultrasound due to soft thinning area of bone on the right frontal scalp.
Figure 2a: Doppler US of right frontal bone show 1 cm hypo echoic mass (circle) without flow and show the defect in the internal part of diplopic of bone (arrow).
Figure 2b: Axial CT of head (bone window) show the same lytic frontal lesion (circle) with internal bone defect (arrow) was done exision of this unusual frontal presentation of the lesion and histology revealed total osseous atretic meningocele with remnants of meningothelial elements.
The development of atretic cephaloceles and their classification remain a source of controversy. A variety of pathogenetic explanations have been described, including nearly complete resolution of a larger meningoencephalocele formed in early fetal life, persistence of a fetal nuchal bleb caused by early embryonic cerebral "blow-out," and persistence of neural crest remnants [1-7].
Regardless of the mechanism of atretic cephalocele formation, vertical embryonic positioning of the straight sinus has frequently been identified in these lesions and deserves mention both as a marker of the timing of the embryologic insult and as a clue to radiologic diagnosis "hallmark imaging sign". During stage 7a of cranial venous development, corresponding to a crown-rump length of approximately 80 mm, the straight sinus is nearly vertical in a ventral-dorsal plane. At this time, the superior sagittal sinus is in the process of formation, as the result of posterior migration of the primitive transverse sinuses (marginal sinuses) and their subsequent coalescence in the midline. With expansion of the cerebral hemispheres, the straight sinus and tentorium acquire a more horizontal course, reaching the typical adult configuration after approximately the third month of gestation. If a midline fibrous strand were present connecting the mesencephalic tectum to the overlying membranous cranium, it is logical to assume that there could be an interruption of the normal progression of movement of the overlying straight sinus and midline tentorium to the normal adult positioning remaining in vertical position (Figure 3) Similarly, such a strand would need to be accommodated by the developing superior sagittal sinus, coalescing from the paired lateral primitive transverse sinuses. The frequent fenestration or high bifurcation of the superior sagittal sinus is therefore understandable (Figure 4). If the fibrous strand becomes discontinuous during this period of venous development, the straight sinus may achieve normal adult positioning. However, even in this case, a short fibrous stalk may be intimately related to the superior sagittal sinus, a possibility that must be borne in mind at the time of cyst or nodule excision.
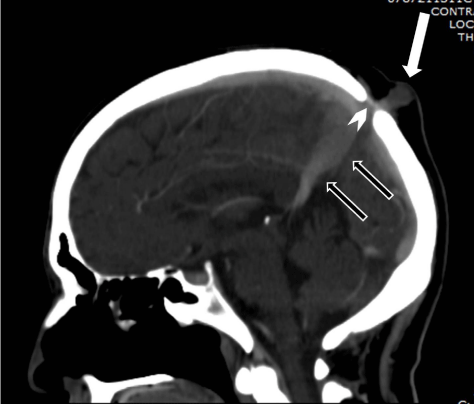
Figure 3: Sagittal reconstruction of contrast CT of head was done to a-53- year old man who is suffering from lung cancer, Showing atretic menigocele (white arrow), vertically positioned straight sinus with persistent fetal anatomy (black arrows) and note also the fibrous tissue that separate the atretic mass from the intracranial structures (arrowhead).
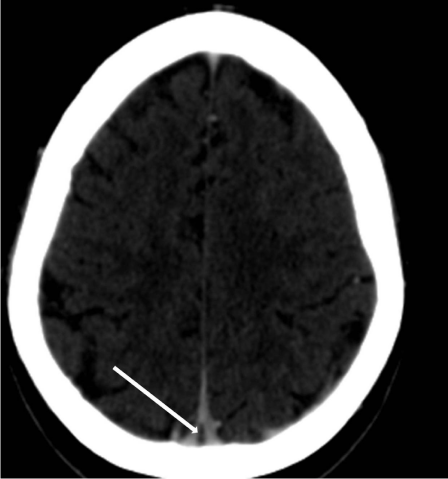
Figure 4: The same patient in figure 3. Fenestration of the superior sagittal sinus (arrow).
Any way the precise timing of formation of the fibrous strand cannot be determined on the basis of venous development other than to say that the stalk predates the appearance of the superior sagittal sinus (at approximately 10 weeks' gestation, stage 7a). Therefore, a number of plausible explanations remain regarding the origin and timing of the embryologic event that culminates in formation of the subscalp cyst/nodule and associated stalk. Perhaps the most compelling is the concept of the "remnant nuchal bleb". Inoue and colleagues have drawn our attention to the work of Ingalls, who noted the relative frequency of small midline scalp cysts in early embryos. The position of these cysts over the developing anterior rhomb encephalon is noteworthy. These cysts could reflect transient over distention of the rhomb encephalic vesicle [6-11].
The prevalence of NTDs varies widely with ethnic and geographic variations, ranging from a high of 3.05 to 6.79 per 1000 live births in the British Isles to a low of 0.1 to 0.6 per 1000 live births in continental Europe and Japan. The frequency of cephaloceles ranges from 1 in 5000 to 1 in 9000 live births and the incidence of atretic cephaloceles is 4-17% of all cephaloceles and the parietal location compromises 40 to 50 % of cases [8-13].
Some described familial recurrence and Caravalho described a genetic predisposition reporting about Autosomal dominant atretic cephalocele with phenotype variability: of a Brazilian family with six affected in four generations [14,15].
Neilan E. reported the presence of a cranial meningocele with Peters anomaly: a balanced paracentric inversion of chromosome 4, inv (4) (q12q13.3) [16,17].
There is a wide range of clinical presentations of patients with atretic cephaloceles. A child may be completely normal with regard to neuro-developmental milestones, or may have severe mental retardation if the atretic cephalocele is associated with severe intracranial anomalies, such as malformations of cortical development, Walker-Warburg syndrome, agenesis of corpus callosum and/or ventriculomegaly. However NTDs are potentially serious congenital malformations. If undiagnosed in childhood, such lesions may later be mistaken for a variety of other soft tissue abnormalities. The proper identification of these lesions is important because NTDs may communicate directly with the central nervous system, and a traumatic injury has the potential to cause a devastating infection. Neuroimaging is of paramount importance in order to make a proper diagnosis [3,17,18].
Ideally, cephaloceles and related lesions are diagnosed prenatally by fetal ultrasonography or shortly after birth. Early diagnosis allows for assessment and evaluation of any associated anomalies [11,19,20].
In infants and children, the focus of the initial examination and neuroradiologic evaluation is the determination of the child's long-term neurologic prognosis. When the condition is discovered during adolescence or adulthood, the focus shifts to making the correct diagnosis and defining the underlying anatomy. The lesions may be discovered incidentally during an examination or after trauma to the head or during computed tomography for staging a malignancy like one of our cases. Although a direct blow to the lesion could theoretically cause it to rupture, a laceration contiguous to the NTD presents the highest potential for infection. If a person with an NTD has grown past childhood without developing any neurologic sequelae, he or she is at low risk for future neurologic problems [12,20].
Scalp lesions similar to our patient's can be difficult to diagnose without imaging. The differential diagnoses of such a lesion without imaging are:
Dermoid cysts: are made up of epithelial cells, sweat glands, or hairs forming subcutaneous collections. They are typically nontender, firm, and no compressible and have normal overlying skin. None of these characteristics were apparent with our patient's lesion.
Hemangiomas: are collections of blood vessels just below the skin. They are rarely tender and are typically dark blue or red in appearance.
Lipomas: are nontender, subcutaneous collections of fat cells. They are deep in the dermis, and the overlying skin glides smoothly over the nodule with palpation. Sebaceous nevi and aplasia cutis congenita: are hairless lesions, each presenting with a bald patch of skin. Sebaceous nevi are a yellow-orange color and become bumpy with new growth as the child passes into adolescence. The lesion of aplasia cutis congenita represents a developmental anomaly that culminates in the loss of dermis, epidermis, fat, or all overlying skin tissue and is usually quite distinctive [13,20,21].
Scalp lesions may also be confused with recent trauma or scar tissue. The location and associated findings, such as skin dimpling and increased concentration of hair follicles (hypertrichosis), should steer the examiner toward a broader differential diagnosis. In general, hypertrichosis, dimples, lipomas, hemangiomas, or cysts overlying the spinal column or the midline of the skull should raise suspicion for underlying neurologic abnormalities. A palpable skull defect should trigger serious concerns and lead to a thorough evaluation. All of these lesions can be excluded by computed tomography examination which can also confirm the diagnosis of atretic meningocele.MRI can be helpful in diagnosing associated anomalies and is recommended when patients are symptomatic [21].
Ultrasound generally is the first modality of imaging for scalp masses and can show a hypo echoic/heterogeneous mass within the skull, continuity of the mass to the intracranial structures must make a suspicious of encephalocele. This is possible because of the bone defect.
A plain skull roentgenogram can generally show a midline bone defect in the parieto-occipital region near the lambda. CT scan can show a high position of the straight sinus. Cerebral angiography can reveal an elongation of the vein of Galen and anomalous upward course of the straight sinus [21].
Although the exact pathogenesis is unknown, but folic acid supplementation has been shown to have a protective effect and is recommended to every woman at the dose of 5 mg daily starting three months before conception.
The prognosis of atretic meningocele is generally good. Of all the reported cases in literature, only one case, which was associated with severe intracranial abnormalities (i.e., moderate hydrocephalus, lissencephaly, and metencephalic hypoplasia), showed profound psychomotor delay; the patient died at 3 years of age [16,20,21].
Other reports of atretic cephalocele, even with associated with grey matter heterotopia, showed normal development. [12,17]. The treatment is surgical excision of the cyst and over sewing of the tract formed by the dura [18,21].
Conclusion
Atretic meningocele is a rare entity of NTDS, usually are asymptomatic, however could be symptomatic when associated with others anomalies. Ultrasound is the first imaging tool. CT is usually diagnostic and MRI is performed only when the patient is symptomatic.
References
- Lumenta CB, Rocco CD, Haase J, Mooij JJA. Neurosurgery. New York, Springer. 2009; 486-490.
- Martinez-Lage JF, Martinez Robledo A, Poza M, Sola J. Familial occurrence of atretic cephaloceles. Pediatr Neurosurg. 1996; 25: 260-264.
- Lemire RJ. Neural tube defects. JAMA. 1988; 259: 558-562.
- Martinez-Lage JF, Sola J, Casas C, Poza M, Almagro MJ, Girona DG. Atretic cephalocele: the tip of the iceberg. J Neurosurg. 1992; 77: 230-235.
- Koester MC, Amundson CL. An Unusual Scalp Lesion in a 15-Year-Old Girl: A Case Report. J Athl Train. 2001; 36: 182-184.
- Davidson L, Gonzalez-Gomez I, McComb JG. Completely intraosseous atretic meningocele. Pediatr Neurosurg. 2009; 45: 308-310.
- Brown MS, Sheridan-Pereira M. Outlook for the child with a cephalocele. Pediatrics. 1992; 90: 914-919.
- Drolet B, Prendiville J, Golden J, Enjolras O, Esterly NB. 'Membranous aplasia cutis' with hair collars. Congenital absence of skin or neuroectodermal defect? Arch Dermatol. 1995; 131: 1427-1431.
- Papanikolaou V, Bibas A, Ferekidis E, Anagnostopoulou S, Xenellis J. Idiopathic temporal bone encephalocele. Skull Base. 2007; 17: 311-316.
- Carvalho DR, Giuliani LR, Simao GN, Santos AC, Pina-Neto JM. Autosomal dominant atretic cephalocele with phenotype variability: Report of a Brazilian family with six affected in four generations. Am J Med Genet Part A. 2006; 140: 1458-1462.
- Neilan E, Pikman Y, Kimonis VE. Peters anomaly in association with multiple midline anomalies and a familial chromosome 4 inversion. Ophthalmic Genet. 2006; 27: 63-65.
- Shane Tubbs R, Elizabeth Hogan, Aman Deep, Martin M Mortazavi, Marios Loukas, Jerry Oakes W. Lateral cephaloceles: case-based update. Child's Nervous System. 2011; 27: 345-347.
- Yokota A, Kajiwara H, Kohchi M, Fuwa I, Wada H. Parietal cephalocele: clinical importance of its atretic form and associated malformations. J Neurosurg. 1988; 69: 545-551.
- Daly S, Scott JM. The prevention of neural tube defects. Curr Opin Obstet Gynecol. 1998; 10: 85-89.
- Peter JC, Sinclair-Smith C, de Villiers JC. Midline dermal sinuses and cysts and their relationship to the central nervous system. Eur J Pediatr Surg. 1991; 1: 73-79.
- Diebler C, Dulac O. Cephaloceles: clinical and neuroradiological appearance. Associated cerebral malformations. Neuroradiology. 1983; 25: 199-216.
- Moron FE, Morriss MC, Jones JJ, Hunter JV. Lumps and bumps on the head in children: use of CT and MR imaging in solving the clinical diagnostic dilemma. Radiographics. 2004; 24: 1655-1674.
- Inoue Y, Hakuba A, Fujitani K, Fukuda T, Nemoto Y, Umekawa T, et al. Occult cranium bifidum. Radiological and surgical findings. Neuroradiology. 1983; 25: 217-223.
- Patterson RJ, Egelhoff JC, Crone KR, Ball WS. Atretic parietal cephaloceles revisited: an enlarging clinical and imaging spectrum? AJNR Am J Neuroradiol. 1998; 19: 791-795.
- Wong SL, Law HL, Tan S. Atretic cephalocele - an uncommon cause of cystic scalp mass. Malays J Med Sci. 2010; 17: 61-63.
- Saatci I, Yelgec S, Aydin K, Akalan N. An atretic parietal cephalocele associated with multiple intracranial and eye anomalies. Neuroradiology. 1998; 40: 812-815.