
Research Article
Austin J Radiol. 2015;2(3): 1017.
Upper Urinary Tract Transitional Cell Tumors Diagnosis: Role of CT- Urography
Recaldini C1, Mangini M1*, De Bon M1, De Chiara M1, Giorlando F1, Duka E1, Marconi A2, Carrafiello G1 and Fugazzola C1
1Department of Radiology, University Hospital of Varese, Italy
2Department of Urology, University Hospital of Varese, Italy
*Corresponding author: Mangini M, Department of Radiology, University Hospital of Varese, V.le Borri 57, 21100, Italy
Received: February 18, 2015; Accepted: March 18, 2015; Published: March 20, 2015
Abstract
Objective: The purpose of this study was to point out CT Urography (CTU) potentials and limitations for the diagnosis of Upper Urinary Tract Transitional Cell Carcinoma (UUT-TCC) and to suggest how and when to use invasive second-line investigations.
Materials and Methods: 66 patients with a suspected UUT-TCC were examined with CTU; 52/66 patients underwent also Retrograde Pielography (RP). Reference standards were histopathology and the 24-month clinical and imaging follow-up. Moreover the T stage of 15 tumors, treated by surgery, was assessed.
Results: 21/66 patients had a final diagnosis of UUT-TCC; CTU showed a sensitivity of 90.5%, a specificity of 84.4%, a Positive Predictive Value (PPV) of 73.1% and a Negative Predictive Value (NPV) of 95%. The overall accuracy of CTU in evaluation of T parameter was 80%.
In the subgroup of 52 patients, CTU and RP showed both a sensitivity of 85.7%, a specificity respectively of 84.4% and 82.2%, a PPV of 46.2% and 42.8%, a NPV of 97.4% and 97.3%. In 7 cases, false positive both at CTU and RP, biopsy allowed a definitive diagnosis of tumor absence. Moreover in 1 case, false negative both at CTU and RP with positive urinary cytology, endoscopy and biopsy showed a small superficial tumor.
Conclusion: CTU, complemented by urinary cytology and cystoscopy, is the technique of choice for UUT-TCC diagnosis and staging. In cases of positive CT findings, RP-guided biopsy is advisable to complete the diagnostic work-up; nevertheless, if CTU is negative and cytology is positive, endoscopy is indicated.
Keywords: Transitional cell carcinoma; CT urography; Retrograde Pielography
Abbreviations
CTU: Computed Tomographic Urography; UUT-TCC: Upper Urinary Tract Transitional Cell Carcinoma; RP: Retrograde Pielography; PPV: Positive Predictive Value; NPV: Negative Predictive Value; IVU: Intravenous Urography; TP: True Positive; TN: True Negative; FP: False Positive; FN: False Negative; RNU: Radical Nephro-Ureterectomy
Introduction
Computed Tomographic Urography (CTU) is currently considered to be the gold standard imaging technique for Upper Urinary Tract Transitional Cell Carcinoma (UUT-TCC) detection [1]. Many studies demonstrated that it is more accurate than Intravenous Urography (IVU) [2,3]. Furthermore, CTU diagnostic accuracy has also been confirmed by a recent literature meta-analysis [4].
However CTU may not detect small superficial urothelial tumors [5], especially if there is incomplete ureteral opacification by contrast medium [6]. In addition, polypoid or stenosing lesions identified with CTU are not always malignant. Therefore the Positive Predictive Value (PPV) of CTU is 53% - 63.2% [7,8], because there are several benign conditions that may simulate tumor [9]. Considering these limitations, the CTU findings must always be supplemented by urine cytology and cystoscopy, if a malignant lesion is suspected. The use of more invasive second-line investigations, such as diagnostic ureteroscopy and Retrograde Pyelography (RP), may be necessary.
The purpose of this study is to point out CTU potentials and limitations for the diagnosis of UUT-TCC; moreover, we aim to suggest when and how to use second line imaging techniques, based on the review of our experience during two years.
Materials and Methods
Study population
Two radiologists (CR and MDB) retrospectively reviewed, in a consensual matter, 92 patients with a suspicion of malignant upper urinary tract tumor, who had undergone CTU and/or RP at our hospital between 1st January 2009 and 31st December 2010. Of these 92 patients, 26 were excluded because of a lack of an established final diagnosis by histhologic examination and/or clinical and imaging follow-up at 24 months.
The remaining 66 patients (51 men and 15 women; age range 41-89 years; mean age 69.4 years) formed the study population (Table 1): 32/66 patients were referred for a positive voided urine cytology for malignant cells (C5: 8 patients), suspicious (C4: 13 patients) or atypical (C3: 11 patients), associated with haematuria (micro or macroscopic) and negative cystoscopy for bladder malignant lesions.
The remaining 34 patients were referred for malignancy because of a new diagnosis of bladder urothelial carcinoma at cystoscopy (7/34 patients, associated with haematuria and positive or suspicious urine cytology), or a prior diagnosis of urothelial tumors (27/34 patients: 23/27 with prior bladder TCC, including 5 patients with recurrent bladder tumor at cystoscopy; 2/27 with prior upper tract TCC; 2/27 with prior bladder and upper urinary tract TCC). The urine cytologic results in the follow-up patient group were: 11/27 positive urine cytology (C5), 6/27 suspicious cytology and 10/27 benign cytology (<C3); moreover 14 of these 27 patients had haematuria.
Fifty-two (52/66, 78.8%) patients underwent both CTU and RP; the mean interval between CTU (performed first in all patients) and RP was of 35.2 days; 14/66 patients (21.2%) underwent only CTU.
The final diagnosis was confirmed by histological examination in 28/66 patients (Table 2). The histological specimens were obtained by surgery in 13 cases (12 nephroureterectomy, 1 ureterectomy), by both biopsy (n=1 case by Ultrasound [US]-guidance; n=3 cases by RP-guidance according to the technique proposed by Carrafiello et al. [10]) and surgery (1 nephrectomy, 3 nephroureterectomy) in 4 cases; by biopsy alone in 9 cases (n=1 case by US-guidance; n=8 cases by RP- guidance), by ureteroscopy with biopsy (with subsequent laser ablation) in 2 cases.
TOT
Surgery
Surgery + biopsy
(pyelographic or ultrasound guidance)
Biopsy
(pyelographic or ultrasound guidance)
Endoscopy
Follow-up
TCC
21
13
3
3
2 *
--
No TCC
45
--
1
6
3
44
TOT
66
13
4
9
5
44
TCC: Tumor Cell Carcinoma.
*2 Biopsies followed by laser ablation.
Table 2: Gold standard of reference in the study population (66 patients).
Pathologically proven TCC was found in 21/28 patients. Histological examination was TCC negative in 7/28 patients (n=6 by biopsy; n=1 by biopsy and surgery); clinical and imaging follow-up, carried out for at least 24 months in 6/7 patients that did not have surgery, confirmed the absence of UUT-TCC.
The remaining 38/66 patients underwent clinical and imaging follow-up for at least 24 months, which demonstrated the absence of UUT-TCC; 3 of them underwent also a diagnostic ureteroscopy, which was negative for tumor (Table 2).
CT urography protocol
All CT exams were performed on a 64-multidetector CT scanner (Aquilon 64, Toshiba Medical Systems, Tokyo, Japan) using a threephase single-bolus protocol. Patients were supine during scanning; all scans were performed from diaphragm to groin. Unenhanced CT was performed in all the patients. Nephrographic phase images were obtained beginning 100 seconds after intravenous non-ionic iodinated contrast medium injection (Iopamiro 300, Bracco, Milano, Italia, at dose of 2ml/kg) at 3 ml/sec injection rate, followed by 40 ml of saline solution (injection rate of 3 ml/sec). Excretory phase images were obtained approximately 12 minutes after the injection of contrast material. Intravenous furosemide (at dose of 0.1 mg/kg) was administered before the contrast medium injection.
The scanning parameters of each phase were 10-500 mA of tube current using current modulation (Sure Exposure) software, 120 kV, 64 x 0,5 mm collimation, and a pitch of 0.828, with smooth reconstruction filter (type 11 and 13).
Five millimetre slices were used for axial plane evaluation; for Multiplanar Reformatting (MPR) and volumetric reconstructions, images were reconstructed with slice thicknesses of 0.5 mm and reconstruction index of 0.3 mm. The following post-processing reconstruction techniques were used: thick-slice Maximum Intensity Projection (MIP), MPR created in true coronal plane passing through the kidney and curved MPR (cMPR). Images were evaluated using raw-data acquired with volumetric scans on axial plane and MPR reconstructions on orthogonal planes (coronal and sagittal).
Retrograde pyelography protocol
All procedures were performed with fluoroscopic guidance on two machines (Allura Xper FD20 and Omnia Diagnost Eleva, Philips Medical Systems, Best, NL), available in our department. The first fluoroscopic system detector had a field of view of 38x30cm and a matrix of 2048x1920; the second system detector had a field of view of 38x31cm and a matrix of 1024x1024.
The correct positioning of the ureteral catheter, previously placed by the urologist during cystoscopy, was verified with radiographic exam. Low osmolar iodinated non-ionic contrast medium was used (Iopamiro 300, Bracco, Milano, Italia), suitably diluted with saline solution (mean quantity 50 ml, range variable according to urinary tract compliance). The contrast medium was injected manually at low pressure, under fluoroscopic control, to obtain an optimal distension of the urinary tract.
Radiograms were taken at different filling degrees, in the various projections (antero-posterior, lateral and oblique). A radioscopic/ radiographic study of the entire urinary tract, with progressive ureteral catheter withdrawal from renal pelvis to the bladder, was performed. Late radiograms, about 5 minute after ureteral catheter removal, were taken to evaluate urinary tract emptying.
Image analysis
The image analysis was performed per patients and per anatomic segments; therefore, according to Wang [2], the upper urinary tract was divided into two anatomic segments (calices and renal pelvis; ureter), for a total of 257 segments in the entire serie studied by CTU and a total of 204 segments in the subgroup studied by CTU and RP.
Suspicious lesions for UUT-TCC at CTU included endoluminal mass (classified considering the mass size into smaller or greater than 1 cm) and focal wall thickening (irregular, eccentric or circumferential, extended for a few centimeters) with contrast enhancement [7,9,11]. Calcifications, oncocalix, hydrocalix, hydroureter and/or hydronephrosis, stipple sign, reduced nephrographic and pyelographic effect were considered other useful findings [12,13]. Moreover, when endoluminal lesions were detected, their density value was assessed in the three different phases (unenhanced, nephrographic and pyelographic phases). In particular, lesions were considered as blood clots or stones when they showed high attenuation on unenhanced images (cut-off density value: for clots >50 HU, for stones >100 HU) and no enhancement on post-contrast images.
Finally, we evaluated the local extension of malignant lesions that were treated with surgery (parameter T of the TNM staging) by assessing possible infiltration of the peritumoral adipose tissue, invasion of renal parenchyma or adjacent organs, thus classifying the lesions in confined (Ta, T1 and T2) and invading (T3 and T4) [14,15].
At RP filling defects, stenosis, caliceal or ureteral “amputation”, hydrocalix or hydroureteronephrosis were considered suspicious for UUT-TCC.
Patients with suspicious imaging findings (at CTU and/or RP), that were histologically proven to be malignant lesions, were included in the True Positive (TP) group. True Negative (TN) group included patients in which negative imaging was confirmed at histologic examination and/or at follow-up which was negative for neoplasia. False Positive (FP) group included patients that had suspicious imaging findings for malignant lesion and negative histologic exam and/or follow-up. False Negative (FN) group contained patients in which imaging did not reveal tumor that was subsequently detected by further diagnostic investigation.
Results
Detection and characterization of UUT-TCC
In our study population, 21/66 patients had a final histopathological diagnosis of UUT-TCC, proven as follow: 12 patients by Radical Nephro-Ureterectomy (RNU) specimens, 1 patient by ureterectomy specimen, 3 patients by biopsy and RNU, 3 patients only by biopsy (2 of them subsequently had chemotherapy and the remaining one underwent only clinical monitoring due to advanced age and high surgery risk), 2 patients by ureteroscopic biopsy (with subsequent laser ablation of the lesion). The other 45/66 patients did not have a tumor.
CTU detected UUT-TCC in 19/21 Patients (TP) (Figure 1, 2), while it failed to detect the tumor in the remaining 2 patients (FN) (Figure 3). CTU detected the absence of tumor in 38 of 45 patients (TN), while in 7 patients CTU showed lesions suspicious for TCC (FP) (Figure 4,5; Table 3). The analysis of the results for CTU compared with the reference standard (histopathology and follow-up) and the image analysis per anatomic segments are summarized in Table 3.
Neoplastic disease
Non neoplastic disease
patients
21
anatomic segments
22
patients
45
anatomic segments
235
CTU
Positive
19
19
7
7
CTU
Negative
2
3
38
228
CTU: per patient sensitivity 90.5%, specificity 84.4%, diagnostic accuracy 86.4%, PPV 73.1% and NPV 95%.
CTU: per anatomic segment sensitivity 86.4%, specificity 97%, diagnostic accuracy 96.1%, PPV 73% and NPV 98.7%.
Table 3: Correlation between CT Urography (CTU) results and histological findings and/or follow-up at 24 months (66 patients and 257 anatomic segments studied with CTU).
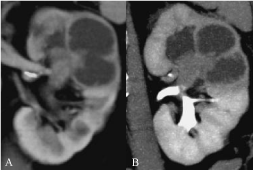
Figure 1: (A) CTU nephrographic phase, coronal MPR: endoluminal mass,
with contrast enhancement, fills the upper pole collecting duct, causing
focal hydronephrosis. (B) CTU excretory phase, coronal MPR: the mass,
suspicious for invading tumor, causes reduction of cortical thickness and
absence of excretory function. Urinary cytology: C5. Histology by RNU: TCC
pT2G3 (CT error of over-staging).
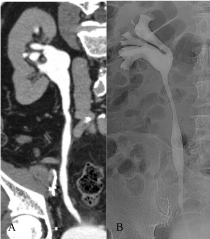
Figure 3: A) CTU excretory phase, cMPR: the exam does not detect any
endoluminal lesion or abnormal wall thickening. B) RP: the exam is negative.
Urine cytology: C5. The diagnostic ureteroscopy identified a small superficial
lesion located at distal ureter: biopsy was positive for TCC and laser ablation
of the lesion was performed.
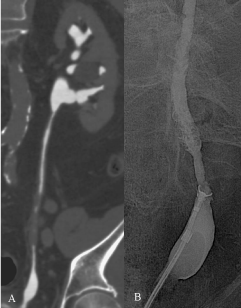
Figure 4: A) CTU excretory phase, cMPR: irregular eccentric ureteric
thickening. Urine cytology: C4. B) RP-guided biopsy with a flexible forceps of
the ureteric structure: the histology finding was chronic inflammation. Followup
at 24 months confirmed the absence of neoplastic disease.
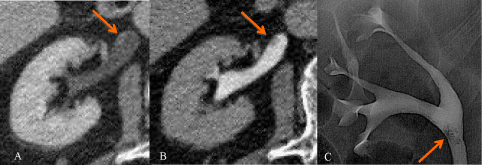
Figure 5: A) CTU nephrographic phase, axial: small (<1cm) endoluminal
mass, with contrast enhancement, on the antero-lateral wall of the renal
pelvis (arrow). B) CTU excretory phase, axial: small filling defect (arrow).
Urinary cytology: C4. C) RP: small filling defect in the renal pelvis (arrow).
Histology by RP-guided biopsy: chronic inflammation. Follow-up at 24 months
confirmed the absence of neoplastic disease.
Fifty-two (52/66) patients underwent both CTU and RP with final diagnosis of UUT-TCC in 7 patients and tumor absence in 45 patients (Table 4). In 6/7 patients with UUT-TCC final diagnosis, both imaging techniques were positive (TP) (Figure 2); in the last patient with TCC, both imaging techniques failed to detect the tumor (FN) (Figure 3).
Neoplastic disease
(patients)
7
Non neoplastic disease
(patients)
45
CTU
Positive
Negative
6
1
7
38
RP
Positive
Negative
6
1
8
37
CTU: per patient sensitivity 85.7%, specificity 84.4%, diagnostic accuracy 84.6%, PPV 46.2% and NPV 97.4%.
RP: per patient sensitivity 85.7%, specificity 82.2%, diagnostic accuracy 82.7%, PPV 42.8% and NPV 97.3%.
Table 4: Correlation between CT Urography (CTU) and Retrograde Pielography (RP) results and histological findings and / or follow-up at 24 months (52 patients studied with both techniques).
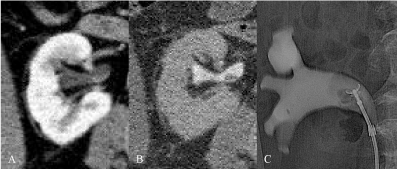
Figure 2: A) CTU nephrografic phase, axial: small (<1cm) endoluminal mass,
with contrast enhancement, on the posterior wall of the renal pelvis. B) CTU
excretory phase, axial: small filling defect. Urinary cytology: C5. C) RP: small
filling defect submitted to biopsy with a flexible forceps (histology: TCC).
Histology by RNU: TCCpTaG2.
Both methods agreed in 44/45 tumor-free patients. In 37/44 cases both techniques showed absence of malignancy (TN); suspicious lesions for TCC were found in 7/44 cases but these were not confirmed by biopsy (FP) and subsequent follow-up (Figure 4,5). CTU and RP disagreed in 1/45 case (FP on RP and TN on CTU): in this case, the patient underwent ureteroscopy, which was negative, and subsequent follow-up confirmed the absence of malignancy (Figure 6). Correlation between CTU and RP results and histological findings and / or follow-up at 24 months is summarized in Table 4.
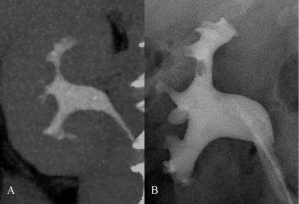
Figure 6: A) CTU nephrographic phase, coronal MIP: the exam does not
identify any endoluminal lesion. B) RP, performed 35 days after the CT exam
and a new episode of haematuria: oval non moving filling defect, localized at
upper pole collecting duct. Urine cytology: C3. The diagnostic ureteroscopy,
performed after RP, did not detect any lesion; therefore the RP finding was
interpreted as a clot. Follow-up at 24 months confirmed the absence of
neoplastic disease.
Overall, in the study population, there were 2 FN cases and 7FP for CTU and 1 FN case and 8FP for RP.
CTU failed to identify in one patient the presence of two malignant ureteral and pelvic lesions, since there was hydronephrosis and a functionally excluded kidney. In this case, with positive urinary cytology, RP should have been useful to evaluate the ureter and renal pelvis; however, this exam was unsuccessful, as the ureteral orifice was occluded by concomitant recurrent bladder tumor. The patient was submitted directly to surgery (radical cystectomy and RNU). The other case, not identified either on CTU and RP, was a superficial lesion, causing no endoluminal defect or wall thickening (Figure 3). Ureteroscopy, performed because urinary cytology was positive, was able to locate the lesion, which was removed by laser ablation.
CTU and RP demonstrated suspicious wall thickening in 5 patients with atypical or suspicious urinary cytology: subsequent biopsy (performed by RP-guidance) found inflammatory tissue (Figure 4); in one FP case, both imaging techniques demonstrated a small (<1cm) filling defect: RP-guided biopsy found chronic inflammatory tissue (Figure 5). The last FP case, common to both imaging techniques, was an infiltrating mass, involving the renal pelvis and calices, with suspicious urinary cytology: US-guided biopsy showed inflammation and hyperplasia; at subsequent nephrectomy the surgical specimen was found to be an inflammatory pseudo tumor.
Finally in one patient, with repeated episodes of gross haematuria and atypical urine cytology, CTU was negative (TN), while RP found a small Filling defect (FP) [Figure 6]: the RP finding was not confirmed by subsequent ureteroscopy and therefore it was attributed to a non moving blood clot. It must be considered that CTU exam was performed 35 days before pyelography: it is likely that CTU, if it had been carried out at the same time as the RP, would have been able to identify and to correctly characterize the clot.
Correlation between CTU results supplemented by secondline investigations and histological findings and/or follow-up at 24 months is summarized in Table 5.
Neoplastic disease
(patients)
21
Non neoplastic disease
(patients)
45
CTU supplemented by
2nd-line investigation
Positive
20 (19 + 1*)
0
CTU supplemented by
2nd-line investigation
Negative
1***
45(38 + 7**)
*: 1 ureteroscopic biopsy positive for TCC
**: 6 RP-guided and 1 US-guided biopsies negative for transitional cell carcinoma
***: 1 RP unsuccessful
Supplemented CTU: per patient sensitivity 95.2%, specificity 100%, diagnostic accuracy 98.5%, PPV 100% and NPV 97.8%.
Table 5: Correlation between CT Urography (CTU) results supplemented by second-line investigations (66 patients) and histological findings and/or follow-up at 24 months.
CT urography local staging of UUT-TCC
We retrospectively staged the local tumor invasion in 15 of the 19 tumors detected by CTU, comparing imaging findings with the histological report on surgical specimens. Three patients were not submitted to surgery due to poor clinical conditions, while in 1 patient laser ablation therapy during ureteroscopy was chosen, as the lesion was single and superficial.
We found correlation between CTU staging and histological T parameter in 12/15 cases (n=8 confined TCC: Figure 7a; n=4 invading TCC: Figure 7b). There was disagreement between CTU and histological findings in 3/15 cases. CTU understaged 1 lesion (localized at the distal ureter), because there was histological evidence of fat tissue invasion (pT3) while CTU described the lesions confined to the muscular layer. CTU over staged 2 cases (both localized at the renal pelvis, pT2 at histological examination: Figure 1b), because a fat tissue plane between the lesion and the surrounding renal parenchyma was not detected. Therefore, the overall accuracy of CTU in prediction of T parameter was 80%, with 6.7% over-staging and 13.3 % under-staging.
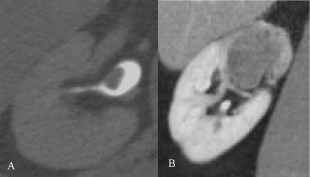
Figure 7: A) CTU excretory phase, axial: mass in the renal pelvis, without
signs of fat invasion; histological staging: TCC pTaG1. B) CTU nephrograpic
phase, coronal: intraluminal lesion occupying the entire upper pole calix and
infiltrating the adjacent parenchyma; histological staging: TCC pT3G2.
Discussion
Since its introduction into clinical practice, IVU was the primary imaging technique for the investigation of urinary tract [16] and it was used as first choice investigation for the patients with haematuria, when upper urinary tract TCC was suspected [17].
However, according to 2011 European Society of Urology guidelines, CTU has become the technique of choice for the UUTTCC investigation, replacing IVU [1]. Thanks to multidetector technology progress, CTU is an excellent imaging modality for urothelial neoplasms identification [2,3,18], with a sensitivity ranging between 88% and 100%, and a specificity between 93% and 100%, as reported in the literature review and meta-analysis by Chlapoutakis et al. [4]. Compared to IVU, CTU has also the advantage of being better accepted by patients, requiring only preliminary fasting without further preparations; it is also quicker and more comfortable than IVU. In addition, CTU is a panoramic examination that allows evaluating urinary tract, renal parenchyma and other abdominal structures in the same session.
In our study, CTU correctly identified 19/21 cases of TCC (TP). In 38/45 cancer free cases CTU was negative (TN), while in 7/45 cases CTU detected suspicious lesions: our FP cases have been caused by inflammatory thickening (n=5), small endoluminal mass (n=1) and inflammatory pseudotumor (n=1), radiologically indistinguishable from cancer [3]. Per patients sensitivity, specificity, diagnostic accuracy, PPV and NPV for the detection of UUT-TCC were 90.5%, 84.4%, 86.4%, 73.1% and 95% respectively. Considering data by anatomic segments according to Wang [2], our results were slightly different (sensitivity: 86.4%, specificity: 97%, diagnostic accuracy: 96.1%, PPV: 73% and NPV: 98.7%): there is particularly an increase of specificity and accuracy.
Our not optimum PPV – which confirms previous results reported in literature [7,8] - is due to chronic inflammation inducing a focal thickening of the wall of the ureter or renal pelvis; in addition several benign diseases - such as fibroepithelial polyp, nephrogenic adenoma, inflammatory pseudotumor - may mimic transitional tumor: in these cases the definitive diagnosis can be established only by histologic examination [9].
Pielography has high sensitivity and specificity (96% and 97% respectively) for UUT-TCC diagnosis, with a PPV of 87% and a NPV of 97%: these values are similar to those of CTU (sensitivity 97%; specificity 93%, PPV 79%, NPV 99%), as reported in the only published study that compared CTU and RP [6]. This procedure, furthermore, allows selective collection of a urine sample for cytological examination [14]. RP, finally, may guide urological forceps biopsy, as described by Carrafiello et al [10], as an alternative to more complex ureteroscopic biopsy, performed in surgery room.
In our experience, 52 patients underwent both CTU and RP: in this group the two imaging techniques produced the same sensitivity (85,7%) and a similar specificity (for CTU 84.4%, for RP 82.2%), as well as similar PPV (46.2% and 42.2%) and NPV (97.4% and 97.3%). In 51/52 patients the two tecniques agreed on probable malignancy (13 patients) or probable benignity (38 patients) diagnosis. The two techniques differed in a single case, with a correct diagnosis for CTU (TN) and wrong diagnosis for RP (FP) (Figure 6). Our results are lower than those reported by Cowan et al. [6], especially PPV.
If we compare our entire series (66 patients) and that of patients who underwent CTU and RP (52 patients), it emerges that only 7/21 UUT-TCCs underwent both imaging studies; the remaining 14/21 UUT-TCCs underwent only CTU, which was considered adequate - in combination with voided urinary cytology and cystoscopy - to conclude the diagnostic work-flow in 12 cases, because suspicious CTU findings - corroborated by positive or suspicious urinary cytology - were highly indicative of upper urinary cancer [7]; in one case RP was unsuccessful, as the ureteral orifice was occluded by concomitant recurrent bladder tumor, while in the other case ureteroscopic-guided biopsy of a mass smaller than 1cm, followed by laser ablation, was added. In the group of seven patients submitted to both examinations, RP - used with only diagnostic aim - did not add any useful information compared to CTU; however, in two small masses and one focal wall thickening – indeterminate at CTU – RPguided biopsy confirmed the tumor presence.
On the other hand, all 45 patients negative for UUT-TCC underwent both CTU and RP, because CTU was never considered sufficient to rule out the suspicion of malignancy. Also in this group diagnostic RP did not add any information compared to CTU; however, in 6 cases in which CTU findings were suspicious, RPguided biopsy allowed a diagnosis of tumor absence (Figure 4,5); in another case – although US-guided biopsy of a kidney mass was negative for neoplastic cell – nephrectomy was performed with inflammatory pseudotumor removal, nevertheless avoiding RNU.
Therefore, RP should be performed only when CTU is inconclusive – that is in cases of incomplete ureteric opacification or of functionally excluded and inadequately excreting kidneys, as well as if intravenous iodinated contrast medium is contraindicated [6,19]; moreover, when CT shows suspicious findings which are not corroborated by positive urinary cytology, second-line investigations (RP-guided biopsy as well as upper urinary tract endoscopy) are useful for characterization of the lesions detected by CT [10].
In spite of high CTU and RP NPV, it should be noted that Carcinoma In Situ (CIS) – neoplastic flat lesion of the urothelium and the basal membrane [1,20] - generally may not be identified with radiographic tecniques [3]; however, CIS is often associated with positive urinary cytology, which is highly sensitive (> 90%) [21,22]. If urine cytology is positive or suspicious and CTU is negative, there is need for upper urinary tract endoscopy, performing selective cytology and biopsy [5,23]. This event happened in one our case – negative at both CTU and RP (Figure 3) - in which RP-guided biopsy was not performed, since absence of abnormal findings did not provide indications for sampling site. Other two patients – with negative imaging studies (CTU and RP) and positive urinary cytology – underwent ureteroscopy that was also negative; they were both tumor-free at follow-up. Nevertheless, if CTU is negative and urinary cytology is negative or atypical, there is a very low UUT-TCC probability [7], so the use of second-line investigations is not justified and patients can undergo the clinical and instrumental follow-up.
To sum up, second line investigations should be used only in selective cases after CTU, to improve its diagnostic performances: in our study sensitivity increased from 90.5% to 95.2%, specificity from 84.4% to 100%; diagnostic accuracy from 86.4% to 98.5%; PPV from 73.1% to 100%; NPV from 95% to 97,8%.
Accurate preoperative staging is important for the proper management of the patient: CTU, compared to conventional methods (IVU and RP), allows a contemporary study of the urinary tract and of the whole abdomen, to assess the extension of disease with a single examination.
However, CTU is not reliable to differentiate between superficial tumor, in which the lamina propria of the mucosa is not invaded (Ta), and tumor extended to subepithelial connective tissue (T1) or to muscularis (T2). Moreover, there are some limits in distinction between early stage (Ta-T2) and advanced one (T3-T4) [11,15,24]: the distinction between T2 (tumor confined to the muscularis) and T3 (tumor extended beyond the muscularis) is not always feasible, since the microscopic infiltration of extra-parietal fat is not detectable with currently available imaging techniques; in addition, tumor may sometimes cause an extra-parietal inflammatory reaction, which mimics the neoplastic infiltration and causes an error of CT overstaging [15].
We retrospectively compared CT images with T parameter on the surgical specimen, and evaluated correctly tumour stage in 80% of the cases (12/15 observations): we did not recognize peri-tumoral fat tissue micro invasion in one pT3 lesion, and we overestimated two pT2 lesions because of the presence of inflammatory desmoplasia induced by the tumor; our results are slightly lower than those reported by Fritz et al. (87.8%) [15], who assessed a larger number of cases (41 UUT-TCCs in 39 patients).
Several limitations of our study have to be considered: our series is numerically limited and not homogeneous, because it includes three patient kinds (as reported in Table 1) and not every case was managed according to the same diagnostic work flow; however it is the result of a mono-centric experience, acquired through a 2 year long period. The study, likewise others reported in literature [2,3], is a retrospective data analysis, proposing to discriminate patients with- from those without neoplastic disease. Moreover, the 24 month follow-up may not be sufficient, but other published works [2,3,25] have used a shorter follow-up (12 months).
Haematuria
Cystoscopy
Urine cytology
+
-
+
-
C5
C4
C3
<C3
I group (32 patients)
(1st event of haematuria)
32
--
--
32
8
13
11
--
II group (7 patients)
(staging in new diagnosis of bladder TCC)
7
--
7
--
5
2
--
--
III group (27 patients)
(Follow-up in prior TCC)
14
13
5
22
11
6
--
10
TCC: Tumor Cell Carcinoma.
Table 1: Study population (66 patients).
Conclusion
In conclusion, CTU – complemented by urinary cytology and cystoscopy – is the technique of choice for UUT-TCC diagnosis [1]; RP should be performed only when CTU is inconclusive.
If CT findings, corroborated by positive urinary cytology and negative cystoscopy, suggest a cancer, patients may be submitted to the most appropriate treatment, also thanks to CT staging. In case of positive CT findings, with suspicious or atypical urinary cytology (without bladder lesions at cystoscopy), second-line investigations are indicated for lesion characterization; if urinary cytology is negative, they are optional, because the patients may be directly addressed to the follow-up.
In case of negative CT findings, urinary cytology plays an important role: if it is positive or suspicious (without bladder lesions at cystoscopy), upper urinary tract endoscopy is suggested, to search for a superficial carcinoma, otherwise not detectable; in case of atypical or negative cytology there is a low UUT-TCC probability, so the patients can undergo the follow-up.
References
- Roupret M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011; 59: 584-594.
- Wang LJ, Wong YC, Huang CC, Wu CH, Hung SC, Chen HW. Multidetector computerized tomography urography is more accurate than excretory urography for diagnosing transitional cell carcinoma of the upper urinary tract in adults with hematuria. J Urol. 2010; 183: 48-55.
- Jinzaki M, Matsumoto K, Kikuchi E, Sato K, Horiguchi Y, Nishiwaki Y, et al. Comparison of CT urography and excretory urography in the detection and localization of urothelial carcinoma of the upper urinary tract. AJR Am J Roentgenol. 2011; 196: 1102-1109.
- Chlapoutakis K, Theocharopoulos N, Yarmenitis S, Damilakis J. Performance of computed tomographic urography in diagnosis of upper urinary tract urothelial carcinoma, in patients presenting with hematuria: Systematic review and meta-analysis. Eur J Radiol. 2010; 73: 334-338.
- Wang LJ, Wong YC, Ng KF, Chuang CK, Lee SY, Wan YL. Tumor characteristics of urothelial carcinoma on multidetector computerized tomography urography. J Urol. 2010; 183: 2154-2160.
- Cowan NC, Turney BW, Taylor NJ, McCarthy CL, Crew JP. Multidetector computed tomography urography for diagnosing upper urinary tract urothelial tumour. BJU Int. 2007; 99: 1363-1370.
- Sadow CA, Wheeler SC, Kim J, Ohno-Machado L, Silverman SG. Positive predictive value of CT urography in the evaluation of upper tract urothelial cancer. AJR Am J Roentgenol. 2010; 195: 337-343.
- Xu AD, Ng CS, Kamat A, Grossman HB, Dinney C, Sandler CM. Significance of upper urinary tract urothelial thickening and filling defect seen on MDCT urography in patients with a history of urothelial neoplasms. AJR Am J Roentgenol. 2010; 195: 959-965.
- Wang J, Wang H, Tang G, Hou Z, Wang G. Transitional cell carcinoma of upper urinary tract vs. benign lesions: distinctive MSCT features. Abdom Imaging. 2009; 34: 94-106.
- Carrafiello G, Fontana F, Mangini M, Ierardi AM, Cotta E, Piacentino F, et al. Upper urinary tract biopsy: an old device for a new approach. Radiol Med. 2012; 117: 1152-1160.
- Kawamoto S, Horton KM, Fishman EK. Transitional cell neoplasm of the upper urinary tract: evaluation with MDCT. AJR Am J Roentgenol. 2008; 191: 416-422.
- Wong-You-Cheong JJ, Wagner BJ, Davis CJ. Transitional cell carcinoma of the urinary tract: radiologic-pathologic correlation. Radiographics. 1998; 18: 123-142.
- Browne RF, Meehan CP, Colville J, Power R, Torreggiani WC. Transitional cell carcinoma of the upper urinary tract: spectrum of imaging findings. Radiographics. 2005; 25: 1609-1627.
- Vikram R, Sandler CM, Ng CS. Imaging and staging of transitional cell carcinoma: part 2, upper urinary tract. AJR. 2009; 192: 1488-1493.
- Fritz GA, Schoellnast H, Deutschmann HA, Quehenberger F, Tillich M. Multiphasic multidetector-row CT (MDCT) in detection and staging of transitional cell carcinomas of the upper urinary tract. Eur Radiol. 2006; 16: 1244-1252.
- Dalla Palma L. What is left of i.v. urography? Eur Radiol. 2001; 11: 931-939.
- Oosterlinck W, Solsona E, van der Meijden AP, Sylvester R, Bohle A, Rintala E, et al. European Association of Urology. EAU guidelines on diagnosis and treatment of upper urinary tract transitional cell carcinoma. Eur Urol. 2004; 46: 147-154.
- Van Der Molen AJ, Cowan NC, Mueller-Lisse UG, Nolte-Ernsting CC, Takahashi S, Cohan RH. CT Urography Working Group of the European Society of Urogenital Radiology (ESUR). CT urography: definition, indications and techniques. A guideline for clinical practice. Eur Radiol. 2008; 18: 4-17.
- Lee KS, Zeikus E, DeWolf WC, Rofsky NM, Pedrosa I. MR urography versus retrograde pyelography/ureteroscopy for the exclusion of upper urinary tract malignancy. Clin Radiol. 2010; 65: 185-192.
- Verhoest G, Shariat SF, Chromecki TF, Raman JD, Margulis V, Novara G, et al. Predictive factors of recurrence and survival of upper tract urothelial carcinomas. World J Urol. 2011; 29: 495-501.
- Patschan O, Horstmann M, Thomas C, Schlemmer HP, Stenzl A. [Diagnostic procedures in upper urinary tract urothelial carcinoma]. Urologe A. 2008; 47: 1487-1496.
- Gonzalez-Peramato P, Jimenez-Heffernan JA, Garcia-Gonzalez R, Garcia-Navas R. [Urinary cytology in the diagnosis of upper urinary tract urothelial tumor]. Arch Esp Urol. 2004; 57: 227-238.
- van der Meijden AP, Sylvester R, Oosterlinck W, Solsona E, Boehle A, Lobel B, et al. EAU Working Party on Non Muscle Invasive Bladder Cancer . EAU guidelines on the diagnosis and treatment of urothelial carcinoma in situ. Eur Urol. 2005; 48: 363-371.
- Kirkali Z, Tuzel E. Transitional cell carcinoma of the ureter and renal pelvis. Crit Rev Oncol Hematol. 2003; 47: 155-169.
- Martingano P, Stacul F, Cavallaro M, Casagrande F, Cernic S, Belgrano M, et al. 64-Slice CT urography: 30 months of clinical experience. Radiol Med. 2010; 115: 920-935.