
Case Report
Austin J Radiol. 2015;2(4): 1022.
Giant Retroperitoneal Neuroblastoma in a Teenager Demonstrated by Split-Bolus MDCT
Michele Scialpi1, Raffaele Schiavone2, Antonio Basilicata3, Antonella Tondo4, Irene Piscioli5, Luca Roncati6 and Teresa Pusiol7*
1Department of Surgical and Biomedical Sciences, University of Perugia, Italy
2Meyer Pediatric Hospital, Division of Radiology, Italy
3Pausilipon Pediatric Hospital, Division of Radiology, Italy
4Meyer Pediatric Hospital, Division of Oncohematology, Italy
5Budrio Hospital, Division of Radiology, Italy
6Department of Diagnostic and Clinical Medicine and of Public Health, University of Modena and Reggio Emilia, Italy
7Provincial Health Care Services, Santa Maria del Carmine Hospital, Italy
*Corresponding author: Teresa Pusiol, Provincial Health Care Services, Institute of Pathology, Santa Maria del Carmine Hospital, Rovereto (TN), Italy
Received: February 17, 2015; Accepted: May 02, 2015;Published: May 11, 2015
Abstract
Neuroblastoma represents the most common extra cranial solid tumor among the childhood malignancies. It is rare in adolescents and very rare in adults. Neuroblastoma occurs most commonly in the abdomen, rather than in thorax. For the first time in literature, we have demonstrated the occurrence of a giant retroperitoneal Neuroblastoma in a 14-year-old young teen, by split-bolus Multidetector-Row Computer Tomography (MDCT). This innovative technique, based on the splitting in two boluses of the intravenous contrast medium, combines two phases in a single scan, with significant reduction of the radiation dose.
Keywords: Neuroblastoma; Retroperitoneal tumor; Multidetector computer tomography (MDCT); Split-bolus technique
Introduction
Neuroblastoma represents the most common extra cranial solid tumor among the childhood malignancies. It is the third most common tumor after leukemia and brain malignancies, accounting for about 15% of childhood cancer-related deaths and in the 50% of cases is already metastatic at time of diagnosis [1]. Neuroblastoma is rare in adolescents and very rare in adults and this explains the small series and limited number of reports on the topic in literature. The great majority (88.5%) of cases is seen in infancy, while the age at diagnosis exceeds the 5 years in the 10% of patients and the 14 years in only the 1.5% [2]. The tumor has a neuroectodermic origin and it occurs most commonly in the abdomen, rather than in thorax. The intra-abdominal specific sites of its origin are: adrenal glands (35%), retro peritoneum (30-35%), Zuckerkandl’s organ, coeliac axes, paravertebral sympathetic chains [3]. The clinical presentation of abdominal neuroblastoma is usually characterized by discomfort and distension, due to local mass effect. It can be the cause of syndromic conditions, such as Hutchinson syndrome (pain and limping due to skeletal metastases), Pepper syndrome (hepatomegaly due to liver metastases), blueberry muffin syndrome (purpura due to extramedullary hematopoiesis in course of metastatic disease) and dancing eyes - dancing feet syndrome (opsomyoclonus) [4]. Abdominal Computed Tomography (CT) typically shows a heterogeneous mass, with calcifications and necrotic areas of low attenuation, and it allows an accurate TNM staging [1]. We have implemented an innovative split-bolus protocol for the TNM staging by 64-detector row CT, in order to ensure diagnostic accuracy and to obtain reduction of the radiation dose in oncologic patients [5,6]. Here, we report the value of this novel split-bolus MDCT technique in the assessment of retroperitoneal Neuroblastoma in a 14-year-old young teen.
Case Presentation
A 14-year-old young teen was admitted to the hospital for an abdominal palpable mass associated with discomfort, worsened in the last two weeks. The laboratory analyses revealed high serum levels of neuron specific enolase (NSE, 312 μg/L), Lactate Dehydrogenase (LDH, 930 U/L) and ferritin (754 μμg/L), together with a high urinary concentration of vanillylmandelic acid (VMA, 214 mg/24h) and Homovanillic Acid (HVA, 415 mg/24h). After a preliminary Ultrasound (US), which showed a voluminous heterogeneous mass containing calcifications at the left upper abdomen, a split-bolus MDCT of the chest and abdomen by 64-detector row scanner was performed.
Method
Split-bolus MDCT is an innovative method that in a single pass combines arterial and venous phases, allowing the detection of hypo- or hyper-vascular and mixed lesions, together with related lymph node involvement and distant metastases. For a 45-Kg patient, the split-bolus MDCT protocol is based on a single acquisition of the chest-abdomen-pelvis after intravenous injection of 90 mL of contrast medium (Iopamiro 350 mg/mL; Bracco, Milano, Italy), splitted by an automatic power injector (Medrad Stellant, Indianola, PA, USA) into two boluses, as reported schematically in Figure 1. The first bolus consisted in the injection of 54 mL of contrast material at 1.3 mL/ sec, followed by 15 ml of saline solution at same flow rate, in order to obtain an adequate parenchymal and venous system enhancement. The second bolus, which consisted of 36 mL of contrast material at 1.3 mL/sec followed by 15 ml of saline solution at the same flow rate, was injected to obtain late arterial phase. A manual bolus tracking was set up, raising the threshold value at 500 HU, by placing a circular Region of Interest (ROI) in the descending aorta. The scan was cranio-caudally performed, starting from the pulmonary apex toward the pubic symphysis, after a 6-second delay from the arrival of the contrast material in aorta. The inherent delay in the bolus tracking was necessary to move the scan table, give breath-hold instructions to the patient, and tune the gantry parameters. For the split-bolus MDCT protocol, the following acquisition parameters were used: gantry rotation speed 0.75 seconds; slice thickness 2.5 mm; reconstruction index 1.25; pitch 0,935:1; tube voltage 120 kVp with automatic tube current (mA) using z-axis modulation. The examination was completed with axial, coronal and sagittal Multiplanar Reconstructions (MPR).
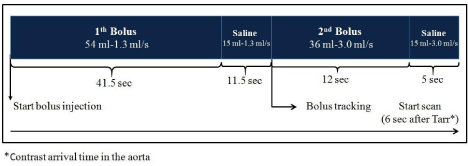
Figure 1: Procedural scheme of the split-bolus whole body MDCT in our 45-
Kg patient, resulting in a simultaneous contrast enhancement of the arterial
and venous system. The volume of the contrast material is calculated as 2
mL/Kg, with a maximum of 90 mL. First bolus, at the start of bolus injection
(or time zero): 54 mL of contrast material at 1.3 mL/sec, followed by 15
ml of saline solution at same flow rate, is injected to obtain an adequate
parenchymal and venous enhancement. Second bolus: 36 mL of contrast
material at 1.3 mL/sec followed by 15 ml of saline solution at the same flow
rate is injected to obtain the late arterial phase.
The split-bolus CT showed a left-sided retroperitoneal heterogeneous mass (11 cm in maximum size) containing calcifications, which compressed and displaced the adjacent organs. No infiltration of the nearby structures or lymph nodes enlargement was revealed (Figure 2A, 2B & 2C). The dose length product (DLP) was 98.1 mGy.cm. The final diagnosis of Neuroblastoma was achieved by the histological examination of the tumor resection specimens (Figure 3).
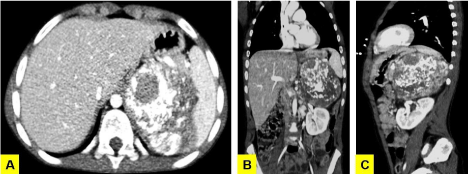
Figure 2: Split-bolus whole body MDCT: the axial (A), coronal (B) and sagittal
(C) multiplanar reconstructions show a retroperitoneal mass in the left upper
side. The voluminous neoplasia is heterogeneous with diffuse calcifications
and defined contours. Note the compression and displacement of the
neighboring structures and vessels by the mass.
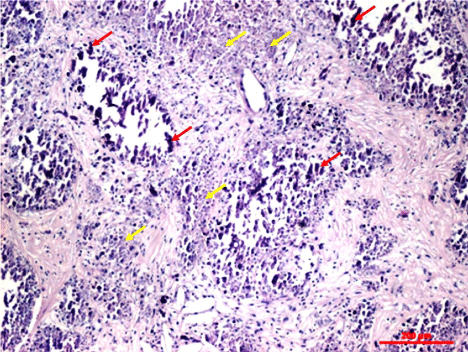
Figure 3: Histologic capture of the giant retroperitoneal neuroblastoma:
the atypical neoplastic nests, at the center of the mass, have undergone a
necrotic process, with consequent deposit of calcium salts. The red arrows
point out the atypical neoplastic cells, while the yellow ones the necrotic areas
(hematoxylin/eosin stain).
Discussion
In the clinical radiologic practice, in order to detect and to define the extent of an abdominal neuroblastoma, US, CT and Magnetic Resonance Imaging (MRI) are used. US usually show a heterogeneous mass with internal vascularity, but also areas of low echogenicity, due to necrosis or calcifications, can be revealed [7]. MRI commonly demonstrates a heterogeneous iso-hypointense mass at T1-weighted images, variable and heterogeneous enhancement, after intravenous administration of Gadolinium (Gd) with Diethylenetriaminepentaacetic Acid (DTPA), and hyperintense cystic/necrotic areas at T2-weighted images [8]. However, the staging of the abdominal neuroblastoma, as reported by International Society of Pediatric Oncology Europe Neuroblastoma Group (SIOPEN), differentiates the locoregional tumors in resectable or unresectable on the basis of a series of ‘Imagine-Defined Risk Factors’ (IDRF), detected by CT [9]. In fact, the CT is the only high-definition technique able to investigate the bone structures, and their relationship with the tumor, and to highlight the intra-tumoral calcifications. On CT the 80- 90% of cases of abdominal Neuroblastoma present some hypodense areas of necrosis into a mass, which insinuates itself beneath the aorta and lifts it off the vertebral column. Moreover, the mass tends to displace adjacent organs, encasing or compressing the nearby vessels. In the most aggressive forms, the lymph node enlargement and the infiltration of psoas muscle or kidney can be revealed [10]. In this last case, the differential diagnosis with Wilms tumor can be difficult [7]. Split-bolus intravenous contrast material administration is an innovative multidetector-row CT technique that in a single pass combines arterial and venous phases, allowing the detection of hypo- or hyper-vascular and mixed lesions, together with lymph node involvement and related distant metastases. If compared with the standard bi- or multiphasic CT, the split-bolus MDCT ensures diagnostic accuracy and efficacy, reducing significantly the radiation dose at the same time. Our double split-bolus MDCT protocol, by means of an optimal enhancement of the arterial, venous system and abdominal parenchymas, has allowed the detection of the mass and has clearly depicted its extension and relationship with the neighboring structures for an adequate staging. The split-bolus single pass whole body MDCT resulted in a DLP of 98.1 mGy.cm and in a reduction of the number of data sets, when compared to multiphasic CT.
Conclusion
The split bolus technique is currently used in patients with hematuria, for its specificity and accuracy in the detection of upper urinary tract tumors [11], and in the study of focal liver lesions [5]. It is intended to increase its fields of application, because it aims to reduce the radiation dose with health benefits for pediatric and adult patients in the ALARA (as low as reasonably achievable radiation) era. The presented case supports the rationale of the split bolus MDCT technique, related to its high accuracy in the detection and staging of retroperitoneal Neuroblastoma and to its radiation dose reduction for the young patients.
References
- Woodward PJ, Sohaey R, Kennedy A, Koeller KK. From the archives of the AFIP: a comprehensive review of fetal tumors with pathologic correlation. Radiographics. 2005; 25: 215-242.
- Franks LM, Bollen A, Seeger RC, Stram DO, Matthay KK. Neuroblastoma in adults and adolescents: an indolent course with poor survival. Cancer. 1997; 79: 2028-2035.
- Lonergan GJ, Schwab CM, Suarez ES, Carlson CL. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. Radiographics. 2002; 22: 911-934.
- Farrelly C, Daneman A, Chan HS, Martin DJ. Occult neuroblastoma presenting with opsomyoclonus: utility of computed tomography. AJR Am J Roentgenol. 1984; 142: 807-810.
- Scialpi M, Palumbo B, Pierotti L, Gravante S, Piunno A, Rebonato A, et al. Detection and characterization of focal liver lesions by split-bolus multidetector-row CT: diagnostic accuracy and radiation dose in oncologic patients. Anticancer Res. 2014; 34: 4335-4344.
- Scialpi M, Pierotti L, Gravante S, Piscioli I, Pusiol T, Schiavone R, et al. Split-bolus versus triphasic multidetector-row computed tomography technique in the diagnosis of hepatic focal nodular hyperplasia: a case report. J Med Case Rep. 2014; 8: 425.
- Caiulo VA, Caiulo S, Gargasole C, Chiriacò G, Latini G, Cataldi L, et al. Ultrasound mass screening for congenital anomalies of the kidney and urinary tract. Pediatr Nephrol. 2012; 27: 949-953.
- Nour-Eldin NE, Abdelmonem O, Tawfik AM, Naguib NN, Klingebiel T, Rolle U, et al. Pediatric primary and metastatic neuroblastoma: MRI findings: pictorial review. Magn Reson Imaging. 2012; 30: 893-906.
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009; 27: 289-297.
- Federico SM, Brady SL, Pappo A, Wu J, Mao S, McPherson VJ, et al. The role of chest computed tomography (CT) as a surveillance tool in children with high-risk neuroblastoma. Pediatr Blood Cancer. 2015; 62: 976-981.
- Maheshwari E, O'Malley ME, Ghai S, Staunton M, Massey C. Split-bolus MDCT urography: Upper tract opacification and performance for upper tract tumors in patients with hematuria. AJR Am J Roentgenol. 2010; 194: 453-458.