
Case Report
Austin J Radiol. 2015;2(6): 1032.
Hepatobiliary Imino Diacetic Acid Scan in the Characterization of Spontaneous Bilomas
Sana S Khan*, Spencer Behr, Tianye Liu and Carina Mari Aparici
Department of Radiology and Biomedical Imaging, University of California, USA
*Corresponding author: Sana S Khan, Department of Radiology and Biomedical Imaging, University of California, San Francisco, 185 Berry Street, Lobby 6 Suite 350, San Francisco, CA 94107-0946, USA
Received: May 05, 2015; Accepted: August 27, 2015; Published: August 28, 2015
Abstract
A 64-year old man was presented to the emergency room for a lower extremity edema and cellulitis. Blood tests showed leukocytosis while computed tomography revealed multiple small hepatic fluid collections suspicious for abscesses. The patient developed sepsis after attempting to drain. Final diagnosis by Hepatobiliary Imino Diacetic Acid scan was spontaneous bilomas with iatrogenic septic complication, post-intervention.
Keywords: HIDA scan; Cholescintigraphy; Bilomas; Nuclear medicine
Introduction
Intrahepatic bilomas usually occur after surgical procedures or trauma involving the biliary system [1]. However, there are reported cases of spontaneous bilomas in the literature, with the most suggested contributing factor being an intraductal pressure increase due to obstructive lesions or infarctions on the biliary tree [2]. Sonography and Computed Tomography (CT) are helpful first line noninvasive imaging techniques in identifying and localizing liver lesions. However, a large variety of hepatic fluid collections have overlapping characterization patterns [3]. A more specific diagnostic technique in the initial differential diagnosis is needed.
Case Report
We present the case of a 64-year old male with a history of diffuse Lymphodenopathy (LAD), elevated liver function tests and ampullary mass. An Endoscopic Retrograde Cholongiopacreatography (ERCP) showed atypical lymphoid hyperplasia suspicious for low-grade B cell lymphoma. However, repeat ampulla biopsies were benign. The patient also had an inguinal lymph node biopsy showing atypical T cell proliferation and B cell hyperplasia with negative T and B cell clonality studies.
Patient presented to the ER with lower extremity edema and redness concerning for cellulitis. The patient arrived afebrile with normal vital signs. However a blood test showed leukocytosis up to 17 x 109/L. CT revealed multiple hepatic small fluid collections concerning for abscesses. Interventional radiology drained one of the collections, finding pus-like fluid with a 4+ White Blood Count (WBC); however, no organisms ever grew from the fluid cultures.
Upon arrival to the ward, the patient reported chills and malaise. He was febrile to 39.4 °C, tachycardic to 120 bpm, and hypertensive up to 170 mm Hg systolic. The patient denied having nausea, vomiting, abdominal pain, diarrhea, constipation, dysuria, hematuria or abnormal discharge. The patient’s WBC was up to 58 x 109/L, meeting sepsis criteria. Blood and urine cultures were negative throughout patient’s hospitalization. Labs showed elevated alanine transminase, aspartate transminase, alkaline phosphatase, direct bilirubin and total bilirubin. Repeat CT reported non hyperenhancing numerous small liver masses and new perihepatic trace ascites. Comparison to a 5-year prior CT showed re-demonstration of the same liver fluid collections. Additional Magnetic Resonance Cholangiopancreaticgraphy (MRCP) was ordered, showing no biliary dilatation, stones, pancreatic mass or pancreatic duct dilation.
After consultation, Hepatobiliary felt cholangitis was unlikely, and imaging ruled out new biliary stricture or choledocholithiasis. MRCP was considered, but deferred due to the patient’s septic status. Interventional radiology was consulted for additional drainage, however, the masses were considered too small. A Hepatobiliary Imino Diacetic Acid (HIDA) scan was ordered, showing a patent common bile duct, normal gall bladder, decreased liver function, evidence of cholestasis and findings consistent with multiple intrahepatic bilomas. Infectious diseases diagnosed the patient with spontaneous bilomas and iatrogenic septic complication post-intervention. The patient recovered after intravenous treatment with antibiotics.
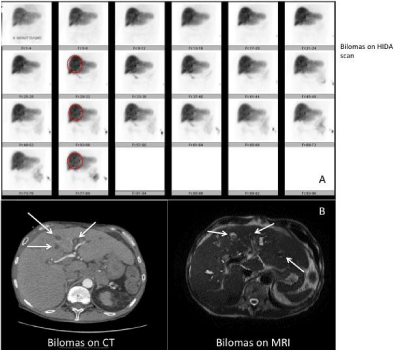
Figure 1: Panel a) shows dynamic HIDA scan images of the patient consistent
with retention of biliary content within the intrahepatic biliary tree, which is
showing multiple spherical shapes consistent with bilomas. The spherical
shapes correlate with the nonspecific hepatic low density lesions identified
on CT and MRI (panel b).
Discussion
Abdominal ultrasound is the first modality to evaluate the nature of a biloma and the underlying pathology [4]. However, a variety of fluid collections in the abdomen have overlapping sonographic patterns and the differential diagnosis is broad. Additional imaging with CT scan shows bilomas as well confined collections with low intraparenchymal or perihepatic attenuation values, a pattern that also shows significant diagnostic overlapping [5]. Differential diagnosis is wide and includes hematoma, seroma, liver abscess, cysts, pseudocysts and lymphocele [6]. Percutaneous aspiration under radiologic guidance can also aid in diagnosis and treatment; however this is an invasive procedure with risks including complications of sedation, drug-related allergic reactions, cardiopulmonary complication, infectious complications, bleeding and non-target access [7].
Standard abdominal T2-weighted MRI or intravenously administered contrast material–enhanced MR imaging could be used for the detection of bile duct dilatations [8]. Although its clinical use is evolving, it is expensive and requires expertise. Artifacts related to MR imaging technique or postprocessing, including incomplete volume acquisition or incorrect reconstruction of a subvolume of ductal data, may create pseudostrictures. Avoidance of these pitfalls requires meticulous attention to both the source images and the postprocessed images. At MR imaging, blooming artifact related to extrabiliary entities such as surgical clips or gas can also create signal voids. Physiologic variants of the biliary system and pulsation or compression artifact from adjacent hepatic arteries has also been implicated in erroneous interpretations of short-segment or bandlike strictures [9]. Although currently, there are three FDA-approved MR contrast agents with some degree of hepatocyte uptake and biliary excretion, they are not community standard [10].
HIDA scans, specifically characterize the biliary system. They are inexpensive, noninvasive and without side effects. They characterize intrahepatic bilomas by demonstrating a photopenic area in the early parenchymal phase that feels-in with activity in the later phase. This differentiates bilomas (spontaneous or post-traumatic) from other lesions that are not part of the biliary tree (hematomas, seromas, cysts, pseudo cysts, lymphoceles or liver abscesses). Caroli disease, a very rare inherited disorder characterized by dilatation of the intrahepatic bile ducts, could have a similar pattern. However, dilations tend to be more tubular and patients present more complex clinical syndromes associated with portal hypertension and congenital hepatic fibrosis [11]. In spite of its simplicity, easy accessibility, specificity and significant image quality improvement with modern Single Positron Emission Tomography (SPECT)/CT, HIDA scans are rarely requested for characterization of liver lesions. Although ultrasound and CT are the standard modality of choice for bilomas, as stated previously, they can cause significant diagnostic overlapping with other diseases that can lead to misdiagnosis. In the case of our patient, the differential diagnosis of the pattern identified on the ordered CT required repeated additional imaging and intervention. This seems to have caused the patient’s sepsis, according to the evaluation conducted by infectious diseases. From this patient’s case, we encourage incorporating HIDA scans to evaluate liver masses when CT or ultrasound fails to provide specific diagnoses. HIDA scans can quickly differentiate lesions related to the biliary system at the early diagnostic stages, and eliminate additional unnecessary and expensive tests. This will result in creating a clear, more narrow diagnosis for prompt treatment, like our case. HIDA scan was the best diagnostic choice for our patient; we highly recommend it be more frequently considered in the future.
References
- Lee JH, Suh JI. A case of infected biloma due to spontaneous intrahepatic biliary rupture. Korean J Intern Med. 2007; 22: 220-224.
- Fujiwara H, Yamamoto M, Takahashi M, Ishida H, Ohashi O, Onoyama H, et al. Spontaneous rupture of an intrahepatic bile duct with biloma treated by percutaneous drainage and endoscopic sphincterotomy. Am J Gastroenterol. 1998; 93: 2282-2284.
- Mueller PR, Ferrucci JT, Simeone JF, Cronan JJ, Wittenberg J, Neff CC, et al. Detection and drainage of bilomas: special considerations. AJR Am J Roentgenol. 1983; 140: 715-720.
- Bas G, Okan I, Sahin M, Eryilmaz R, Isik A. Spontaneous biloma managed with endoscopic retrograde cholangiopancreatography and percutaneous drainage: a case report. J Med Case Rep. 2011; 5: 3.
- Yoon W, Jeong YY, Kim JK, Seo JJ, Lim HS, Shin SS, et al. CT in blunt liver trauma. Radiographics. 2005; 25: 87-104.
- Akhtar MA, Bandyopadhyay D, Montgomery HD, Mahomed A. Spontaneous idiopathic subcapsular biloma. J Hepatobiliary Pancreat Surg. 2007; 14: 579-581.
- Lorenz J, Thomas JL. Complications of percutaneous fluid drainage. Semin Intervent Radiol. 2006; 23: 194-204.
- Nandalur KR, Hussain HK, Weadock WJ, Wamsteker EJ, Johnson TD, Khan AS, et al. Possible biliary disease: diagnostic performance of high-spatial-resolution isotropic 3D T2-weighted MRCP. Radiology. 2008; 249: 883-890.
- Irie H, Honda H, Kuroiwa T, Yoshimitsu K, Aibe H, Shinozaki K, et al. Pitfalls in MR cholangiopancreatographic interpretation. Radiographics. 2001; 21: 23-37.
- Frydrychowicz A, Lubner MG, Brown JJ, Merkle EM, Nagle SK, Rofsky NM, et al. Hepatobiliary MR imaging with gadolinium-based contrast agents. J Magn Reson Imaging. 2012; 35: 492-511.
- Yonum O, Bayraktar Y. Clinical characteristics of caroli’s syndrome. World Journal of Gastroenterology. 2007; 13: 1934-1937.