
Case Report
Austin J Radiol. 2017; 4(3): 1073.
A Case of High Altitude Related Splenic Infarct in a Previously Undiagnosed Sickle Cell Trait
Chinmaya Deepak Patro, Aruna R Patil*, Himansu Mohanty, Kapil Shirodkar and Prabhu Meganathan
Department of Radiology, Consultant Radiologist & Associate Professor, Apollo Hospitals, Bangalore, India
*Corresponding author: Aruna R Patil, Department of Radiology, Consultant Radiologist & Associate Professor, Apollo Hospitals, Bangalore -560078, India
Received: August 01, 2017; Accepted: September 07, 2017; Published: September 26, 2017
Abstract
Sickle cell trait is the heterozygous disease form of sickle cell anemia which is generally asymptomatic. Manifestations such as vasoocclusive crisis occur when patients are subjected to anoxic / hypoxic situation, identification of which is important for understanding the cause and management. Splenic infarct is such a manifestation. In this case report we highlight the importance of considering underlying hemolytic anemia in patients presenting with vasoocclusive crisis such as splenic infarct at high altitude.
Keywords: High altitude; Splenic infarct; Sickle cell trait
Introduction
Sickle cell anemia is one of the common causes of hemolytic anemia. Sickle-cell trait is the heterozygous form of the disease (HbAS). Though this condition remains asymptomatic, manifestations that occur when exposed to hypoxia leads to uncovering of the condition. Splenic infarct is such a manifestation in patients with sickle cell trait at high altitude. In this case report we highlight the importance of considering underlying hemolytic anemia in patients presenting with vasoocclusive crisis such as splenic infarct at high altitude.
Case Report
A 44year old male with no significant co-morbidity had a recent visit to a high altitude spot (~ 11,000 ft above sea level). He developed sudden onset of vague abdominal pain radiating towards the left shoulder. He had 2 to 3 episodes of vomiting. He was then immediately admitted to the local hospital where symptomatic treatment was given. Blood oxygen saturation was recorded as 80%.
On return to lower altitude, blood investigations were done as follows: Hemoglobin: 11.8 gm/dL, RBC count: 4.4 millions/cm3, Reticulocyte count: 2.9%. Liver function tests were abnormal as follows: serum bilirubin: 5.8 mg/dL, serum alkaline phosphatase: 139 U/L, SGOT: 49 U/L, SGPT: 48 U/L, serum GGT: 95 U/L. Malaria parasites were negative.
On ultrasound imaging, spleen was enlarged measuring 15cms. Majority of the spleen was hypoechoic and coarse with no color uptake. Vessels at the hilum appeared normal (Figure 1).
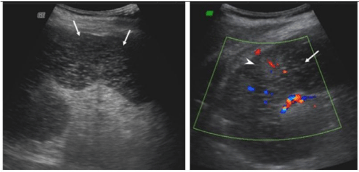
Figure 1: Ultrasonography of the spleen shows predominantly hypoechoic
coarse areas which show absent color uptake (white arrows). Spared areas
(white arrow heads) appear echogenic with preserved color flow.
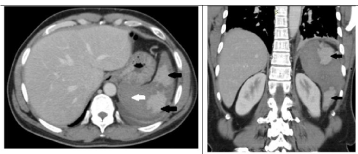
Figure 2: a: Axial and b: Coronal sections of CECT (porto-venous phase)
shows predominantly non enhancing enlarged spleen representing infarct
(white arrow). Enhancing areas (black arrows) are viable tissue. Note left
pleural effusion and minimal perisplenic collection.
On contrast enhanced MDCT, the spleen was enlarged with majority of the splenic parenchyma showing no enhancement with patchy spared areas of normal enhancement. Splenic vessels up to the hilum appeared normal. Minimal perisplenic fluid was seen along with mild left sided pleural effusion (Figure 2a,b). Patient underwent MRI as a part of screening for underlying lymphoproliferative disorders. The infracted spleen was predominantly T2 hyperintense with diffusion restriction and few punctate hyperintense foci on T1 weighted images suggestive of hemorrhage. Thin hemorrhagic perisplenic collection was seen (Figure 3a,b,c).
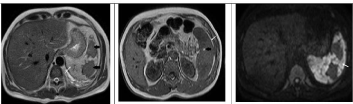
Figure 3: a: Axial T2 weighted MR image shows infarcted portions as hyper intense areas (white arrow) and spared areas in black arrows.
b: Axial T1 weighted MR image shows tiny hemorrhagic foci (black arrow) and thin perisplenic hematoma (white arrow)
c: Diffusion weighted imaging reveals diffusion restriction of the infarcted spleen.
A suspicion of underlying hemolytic process provoked by high altitude related hypoxia was sought. A peripheral smear was done which showed normochromic normocytic anemia. Sickling test showed occasional sickle cells. Hb electrophoresis showed 53.87% Hb A, 42.55% Hb S, 3.57% Hb A2 which was suggestive of sickle cell trait.
Patient underwent splenectomy due to extensive infarct. The gross pathology showed areas of grey white to grey yellow which represented infarcts. Histopathology showed ischemic necrosis of splenic parenchyma involving more than 90%. The necrosis was surrounded by zone of hemorrhage and congestion with mild neutrophilic margination (Figure 4a,b).
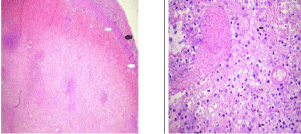
Figure 4: a: Microphotograph (5X magnification, H&E stain) shows diffuse
necrosis of spleen with loss of architecture. Black arrow is the splenic capsule
with white arrows showing subcapsular hemorrhage.
b: Microphotograph (10X magnification, H&E stain) shows infiltration of spleen
with inflammatory cells composed of neutrophils and histiocytes (arrows).
Discussion
Sickle cell disease is a hereditary hemoglobinopathy which results from a point mutation where valine is replaced by glutamic acid at sixth position of beta globin gene of hemoglobin. It is an autosomal recessive disorder. Normal adult hemoglobin has two pairs of alpha and beta chains. About 7% of the world’s population is carrier of some form of hemoglobin disorder [1]. Sickle-cell trait is the heterozygous form of the disease (HbAS). Patients with sickle cell trait inherit normal hemoglobin a gene from one parent and an abnormal hemoglobin S gene from the other parent [2]. In sickle cell trait approximately 40% of hemoglobin is HbS, rest being HbA. So under normal conditions due low concentration of HbS and presence of interfering HbA act to prevent efficient HbS aggregation and polymerization. In hypoxic conditions when the blood oxygen levels are low the sickle Hemoglobin (HbS) molecule is deoxygenated and there is hydrophobic interaction with other HbS molecules that triggers an aggregation into large polymers resulting in sickle shaped deformity of Red Blood Cells (RBC). The deformed RBCs cannot flow freely within the vessels and result in accumulation at the branching point of the vessel which forms the thrombus. The common critical manifestations include vasoocclusive, sequestration, hemolytic and aplastic crisis [3,4]. Sickling is confined to microvasculature beds where blood flow is sluggish. This is normally seen in spleen and bone marrow [2].
Splenic infarcts are expected complications in diseases like myeloproliferative disorders, hemolytic anemia, sepsis, lymphoma and sarcomas. Embolic events like infective endocarditis and atrial fibrillation, abdominal trauma, external compression and torsion can also produce splenic infarcts [5,6,8]. Infarcts may vary in size and may rarely involve the entire organ [7].
Clinically patients present with upper left quadrant pain which may radiate towards the left shoulder. In some cases it may be silent [7]. Patients may develop ipsilateral pleural effusion, perisplenic abscess or splenic vein thrombosis.
Ultrasound is simplest modality available for screening splenic infarct. The infarcted region appears hypo echoic and wedge shaped to the rest of the normal spleen. On color Doppler the infarcted region shows no color uptake [9]. On CT the infarct appears as sharply contoured wedge shaped, hypo dense area without any contrast enhancement [7]. On MRI infarcts show variable signal intensity on both T1- and T2-weighted images based on the time since the vasoocclusive episode and do not enhance after intravenous contrast material administration.
Conservative treatment is the main stay of management .Surgical intervention is required when there is total infarct, sub capsular hemorrhage, hemoperitoneum or abscess formation [9].
Conclusion
This report highlights the fact that underlying dormant hemolytic condition should be suspected in an otherwise normal individual experiencing symptoms, signs of vasoocclusive crisis (in this case – splenic infarct) under hypoxic conditions such as in high altitude ascent [5,10].
References
- Ajit C Gorakshakar. Epidemiology of Sickle Hemoglobin in India. Proceeding of National Symposium on Tribal Health.
- Robbins, Cotran. Pathologic basis of diseases. 7th edition. 2005; 628-631.
- Morishima A, Schofer JM, Pelletier P, McKee JM. Images in Emergency Medicine: Splenic Infarction Due to Sickle Cell Trait after Climbing Mt. Fuji. Western Journal of Emergency Medicine. 2008; 9: 179.
- Sofya H Asfaw, Gavin A Falk, Gareth Morris-Stiff, Ralph J Tuthill, Matthew L Moorman, Michael A Samotowka. “A Unique Cause of Intestinal and Splenic Infarction in a Sickle Cell Trait Patient”. Case Reports in Surgery. 2013.
- Gupta M, Lehl SS, Singh K, Singh R. Acute splenic infarction in a hiker with previously unrecognised sickle cell trait. BMJ Case Rep. 2013.
- Nores M, Phillips EH, Morgenstern L, Hiatt JR. The clinical spectrum of splenic infarction. Am Surg. 1998; 64: 182-188.
- Balcar I, Seltzer SE, Davis S, Geller S. CT patterns of splenic infarction: a clinical and experimental study. Radiology. 1984; 151: 723-729.
- Hoda Zeinab Amer, Channing Chin, Kevin Clarke, Debra Heller. Infarcted ectopic spleen presenting as retroperitoneal mass. Journal of Minimally Invasive Gynecology. 2007; 14: 660-662.
- Goerg C, Schwerk WB. Splenic infarction: sonographic patterns, diagnosis, follow-up, and complications. Radiology. 1990; 174: 803-807.
- Hota PK, Singh KJ. Splenic Infarction: an intriguing and important cause of pain abdomen in high altitude. Bali Med J. 2015; 4: 1-4.