
Review Article
Austin J Radiol. 2018; 5(1): 1081.
Direct Therapeutic Agents Delivery in Ischemic Myocardium: Emphasis on the Role of MRI
Saeed M¹* and Albert K Chin²
¹Department of Radiology and Biomedical Imaging, University of California San Francisco, USA
²Cruzar Medsystems, University of California San Francisco, USA
*Corresponding author: Maythem Saeed, Department of Radiology and Biomedical Imaging, School of Medicine, University of California San Francisco, 185 Berry Street, Suite 350, Campus Box 0946, San Francisco, CA 94107-5705, USA
Received: March 24, 2018; Accepted: April 30, 2018; Published: May 18, 2018
Abstract
Angiogenic growth factors, genes and stem cells have been delivered, during coronary artery bypass grafting, as an alternative treatment, to restore myocytes and blood vessels in end stage patients. It is vital to carefully design, develop, and optimize minimally invasive devices and to use noninvasive diagnostic methods before attempting to apply these devices to clinical routine. Accurate local delivery of therapeutic agents has been recently introduced using endoscopic- and MRI-guidance in experimental animals. Investigators found that local intra-myocardial delivery can be achieved using endoscopic- and MRIguidance.
Minimally invasive methods have the following advantages: 1) it provides direct visual control of the devices in vivo, 2) it allows visualization of infarcted myocardium and precise needle placement in peri-infarct zones during therapeutic cells/gene administration, 3) it provides high retention profile compared to intravenous or intra-arterial routes and 4) it allows intervention lists to avoid damaging coronary arteries/veins or major blood vessels. Furthermore, clinical and preclinical studies have indicated that noninvasive.
MR imaging provides quantitative data on myocardial perfusion, function and viability. Doping of therapeutic agents with MR contrast media is useful for monitoring their distribution in the targets. These capabilities have positioned MR imaging as an important approach to pursue for assessing the benefits of locally delivered therapies. This mini-review addresses our experience and others in delivering local therapeutic agents in ischemic heart disease with special emphasis on the role of MRI in assessing therapies.
Keywords: MR imaging; MRI; Therapeutic agents; Myocardium
Introduction
Ischemic heart disease is a major public and economic health problem. Due to its epidemiologic importance, it became imperative to develop minimally invasive techniques to treat this disease and improve non-invasive diagnostic methods to monitor the efficacy of the treatment. Coronary angioplasty, bypass surgery and thrombolytic therapy are routinely applied to restore blood flow to ischemic myocardium, which has resulted in improvement of Left Ventricular (LV) function. However, many patients with end stage coronary artery disease continue to suffer from disabling angina, risk of LV remodeling, and heart failure. The beneficial effects genes and/ or steam cells has been demonstrated in patients with ischemic heart disease [1,2].
A variety of routes have been used and compared after delivery of genes and/or steam cells therapies (Table 1) [3-7]. The major limitations of these routes are: degradation by blood enzymes, lung entrapment of cells, the need for extremely high doses and poor tissue uptake [8].
Previous Approaches
Recent Approaches
Systemic injection (rare)
MR-guided catheter-based technique
Intra-operative injection during bypass surgery; direct intramyocardial injection (common)
Transthoracic endoscopic technique
Pericardial
Per-adventitial
Percutaneous image-guided catheters;
Transesophageal echocardiography (2D)
Electromechanical mapping technology (NOGA) (scar tissue)
Table 1: Approaches for direct myocardial therapies.
Unlike direct intramyocardial therapy, selective intracoronary infusion enables delivery of therapeutic agents to specific coronary perfusion territory, but this technique provides inefficient retention of drugs in the myocardium. Similarly, genes and cells utilizing large molecules have limited capacity to diffuse into hypoperfused myocardium. It was demonstrated that nano-formulated antiinflammatory drugs in combination with gene product offers a clinical solution to enhance the distribution of drugs in myocardium [9].
In the last decade, investigators focused on the feasibility and effectiveness of direct intramyocardial cell therapy in open chest patients [10-14]. Rosengart et al. indicated that direct intramyocardial therapy is safe and reliable [15]. Several experimental animals explored the direct delivery of therapies and found that direct transfer of angiogenic growth factors, genes and stem cells provides high concentration of therapies in the needed ischemic regions and minimizes the spread of these therapeutic agents to other organ [16- 32]. Vrtovec et al compared the efficacy of transendocardial (n=20 patients) and intracoronary (n=20 patients) delivery of CD34+ cells in non-ischemic dilated cardiomyopathy patients [33]. Investigators found that transendocardial CD34+ cell transplantation is associated with higher myocardial retention rates and greater improvement in ventricular function, and exercise capacity compared with intracoronary route. Others used combined delivery approach (intramyocardial and intracoronary) of bone marrow mononuclear cells at 3-6 weeks and 3-4 months after myocardial infarction in 60 patients using endocardial mapping systems (NOGA-STAR®) [34].
Noninvasive biomedical imaging (positon emission tomography, single-photon emission computed tomography, Magnetic Resonance Imaging (MRI), optical imaging and echocardiography) played an important role in tracking, monitoring and evaluation of the efficacy of cardiac therapies. Cardiologists focused on MRI because it has the potential to chronologically assess left/right ventricular function, myocardial perfusion and viability in treated patients [35,36] (Table 2). MR sequences are also useful for measuring cardiac mass and ventricles geometry, which are used as surrogate endpoints in clinical trials. It can also delineate the complex repair process of infarcted myocardium (edema, necrosis, inflammation and collagen formation [37]. Furthermore, delayed contrast enhanced MRI (DE-MRI) sequences can reliably characterize tissues and measure the size and transmurality of acute and chronic myocardial infarct (Figure 1). After drug delivery, non-invasive MRI provides accurate information on the time course of infarct healing. Summary of MRI sequences used in cardiac imaging are shown in Table 3. Bengel et al. indicated that MRI may be the most suitable noninvasive technique for chronologically monitoring myocardial repair [38].
Tissue characterization
Assessing LV function and perfusion
Molecular imaging
Guiding and monitoring local therapies; genes, stem cells and ablation
Table 2: Applications of MRI in assessing local myocardial therapies.
MR fluoroscopy using fast gradient echo sequences offers a near real-time feedback
Cine MRI for measuring LV volumes, mass and radial strain.
Tagging cine MRI for measuring circumferential stain
Velocity encoded phase contrast cine MRI for measuring longitudinal strain.
First pass MRI perfusion for measuring relative myocardial blood flow.
Delayed contrast enhancement for measuring infarct size.
T1, T2 and T2* mapping
Extracellular volume measurements.
Table 3: MRI sequences for assessing local myocardial therapies.
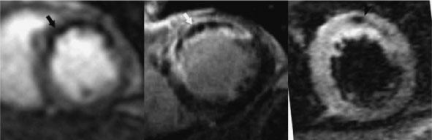
Figure 1: MR images show a reperfused myocardial infarct in the atrioseptal
LV wall of a patient. The territory of perfusion deficit is demonstrated
on the first pass perfusion sequence as a hypoenhanced zone (arrow, left
image). Microvascular zone is also seen as hypoenhanced region on delayed
contrast-enhanced MR image (arrow, center image). On STIR T2-weighted
imaging, the infarct appears bright with hypoenhanced zone (arrow, right
image), suggesting the presence of intramyocardial hemorrhage due to the
damage in microvessels.
The ability to label cells/genes with MR contrast media/tracers prior to local delivery also enhanced the potential to localize and monitor the distribution of administered therapies. The feasibility of endoscopic delivery and MR-guided transendocardial systems for direct transfer of therapies have been demonstrated in experimental animals [39,40]. The advantages of these devices over x-ray-guided intervention procedures are avoidance of radiation, reduction of the incidence of renal damage and allergic reaction induced by iodinated contrast media. Fast MRI-guided acquisition also offers 3-dimential images and functional information in almost real time, thereby, allows interventionist to control the intervention procedures.
Methods of Therapeutic Delivery
Intracoronary delivery
Selective intracoronary infusion enables delivery of therapeutic agents to specific coronary perfusion territory [41,42]. This technique, however, is also limited by inefficient retention of drugs in the myocardium. Thus, investigator introduced coronary vasodilation and vascular/cellular permeability enhancement for dense regional gene transfer [43,44]. Another issue is that genes and cells utilize large molecules that have limited capacity to diffuse throughout the myocardium. It was demonstrated that nano-formulated antiinflammatory drugs in combination with gene product may offer a clinical solution to enhance the distribution of drugs in myocardium [9]. Available data on adeno-associated viruses suggest that vasodilation, vascular permeability enhancement, and avoidance of inhibitors to infection are still relevant variables. Investigators have suggested that slow infusion of a mixture of the adeno-associated viruses, antibodies and contrast media into coronary arteries is an effective means of delivery. However, the recent study by Greenberg et al call into question the efficacy of this open-artery AAV slow infusion approach [45].
Endoscopic-guided device
The configuration of the epicardial injection cannula is shown in Figures 2 and 3. Figure 2 shows an endoscope residing in the lumen of the cannula that contains a specialized distal viewing tip. The transparent asymmetrically conical viewing tip possesses a linear ventral surface. An operating channel resides in the ventral wall of the viewing tip, and this channel accommodates the needle used for intra-myocardial injection. The ventral aspect of the epicardial injection cannula also contains a suction pod that may be used to stabilize the cannula on the epicardial surface of the beating heart, if necessary, during gene injection. Viewing of epicardial morphology occurs as the viewing tip is placed in apposition with the epicardial surface of the heart. The linear configuration of the ventral surface of the viewing tip provides adequate contact with the epicardium to ensure a clear view of the heart. The tissue contact length of the viewing tip against the heart translates into the depth of field of view of the corresponding endoscopic image (Figure 3).
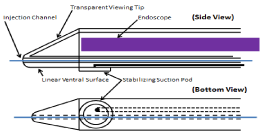
Figure 2: Schematic presentation of two views of the epicardial injection
cannula and port of drug injection. The injection needle length (a) is adjusted
to 7mm.
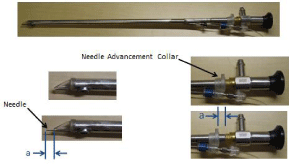
Figure 3: The configuration of the epicardial injection cannula and port of
drug injection. The injection needle length (a) is adjusted to 7mm.
The following surgical technique has been used in our laboratory, where a 2cm skin incision was made in the 4th intercostal space at the anterior axillary line to enter the left pleural cavity in swine (n=24). The endoscopic epicardial cannula was advanced towards the mediastinum. The endoscopic epicardial cannula was advanced further to puncture through the left pleural and pericardial layers at a point midway between the anterior surface of the heart and the posterior aspect of the sternum. Once pericardial entry has been achieved, access is available to both the anterior and posterior aspects of the heart (Figure 4). The endoscopic cannula is initially positioned on the anterior epicardial surface. By moving the cannula inferiorly, the apex of the heart can be visualized. Continued movement of the endoscopic cannula past the apex will position the device on the posterior aspect of the heart. Thus, both anterior and posterior infarcts may be addressed via a single intercostal entry incision. Positioning of the operating channel on the linear ventral surface of the viewing tip provides an unobstructed view of the vast majority of the anatomic field.
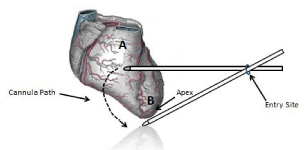
Figure 4: Schematic description of the cannula movement around the heart.
The cannula may access the anterior (A), lateral and around the apex (B) to
the posterior wall of the left ventricle.
As shown in Figure 5, the endoscopic view allows an appreciation of the pericardial sac superiorly, and the epicardial surface, including the outline of the left anterior descending coronary artery. Injection needle exit from the viewing tip occurs at the white dot observed at the distal end of the injection channel.
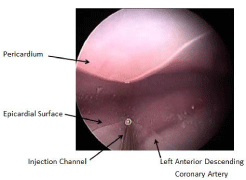
Figure 5: An endoscopic view allows an appreciation of the pericardium
and epicardial surface, including the outline of the left anterior descending
coronary artery.
The main advantages of the endoscopic device are: 1) the endoscopic epicardial injection cannula provides direct visual control of the pericardial entry process; and once the pericardium has been traversed, it imparts guidance for epicardial navigation and myocardial intervention, 2) the epicardial endoscopy allows visualization of infarcted myocardium and precise needle placement in peri-infarct zones during therapeutic cell/gene delivery, 3) this approach eliminates the use of fluoroscopy and 4) direct anatomical viewing assists the clinician in avoiding major coronary artery/ vein injury during myocardial injection and minimizes surgical invasiveness.
The other application of endoscopic device is for ventricular ablation. Ablation of ventricular tachycardia remains a challenge due to the inaccessibility of the epicardial wall from the left ventricular chamber. Schweikert et al found 40% of the patients with ventricular tachycardia require additional epicardial ablation [46]. Zenati et al found that peri-cardioscopic approach is suitable for epicardial ablation and interventions [40].
MR-guided endovascular catheter
Standard MRI sequences are considered a state-of-the-art technique for simultaneously measuring infarct size, regional/global LV function and perfusion in patients. Real-time MRI imaging (A steady state free percession sequence, bFFE) has opened the road toward MRI-guided catheter navigation for delivering local therapies. The endovascular MR-guided catheters were developed by Karmarker et al. [39]. These catheters are equipped with embedded antennae and coils. The properties of an active catheter were tested in vitro using 1.5T XMR scanner (Figure 6) [19]. The bright signal surrounding the catheter allowed visualization of the shaft and the tip, while the external coils are primarily used to define the catheter orientation (Figure 6). The catheter also had dimeters between 9-11F and its distal end incorporates a 27G needle that can be protruded between 3-5mm, depending on the LV wall thickness.
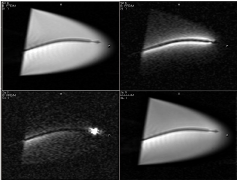
Figure 6: Long-axis MR images acquired in a water bath with different
catheter and external coil elements active. The same acquisition
(FOV=240mm, matrix=160x160, slice=8 mm, TR/TE/flip=4.0ms/2.0ms/700,
2 frames/s) was used in all cases. The top left image shows the activity of
external coils elements, while top right image shows the activity catheter
coil. In the bottom left image, the catheter tip microcoil is active. Bottom right
image demonstrates the activity of all coils elements.
The endovascular catheter was tested in beating hearts and used to deliver tissue markers (mixture of vital dye and MR contrast media) transendocardially. In this study, we found that the contrast media caused myocardial signal enhancement at the tip of the catheter and at postmortem provided evidence that the injection is truly in myocardium (Figure 7).
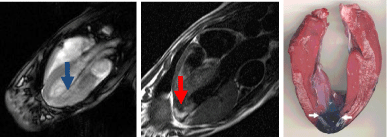
Figure 7: Electrocardiographically gated dual-inversion-recovery T1-
weighted contrast-enhanced MR image (oblique-coronal view, 500/40) shows
the catheter in the LV (arrow, left) and transmural enhancement at the heart
apex (arrow, middle) after administration of 2 mL of a mixture of gadodiamide
and blue dye. MRI of the middle slice shows the close correspondence of the
stained tissue (arrows right) with the enhanced region on MRI.
MRI offers interactive 3D steering of imaging plane, moderate soft tissue contrast and wash in/wish out of injected contrast media. Furthermore, fast MRI acquisition allows guiding and tracking of the catheters in the aorta and LV (Figures 8).
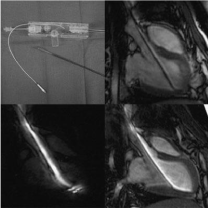
Figure 8: A catheter for MRI-guided intramyocardial injections (top left),
which has been used for passive (top right) or active tracking (bottom). On
the top-right image the catheter coil is switched off and the surface coil is on,
showing the catheter as a dark object. On the bottom left image, the surface
coil is off and the catheter coil is on, providing a bright signal around the shaft
and tip of the catheter. On the bottom-right image both surface and catheter
coils are on, providing a bright signal around the catheter and delineation of
the aorta and entire LV.
Figures 9 and 10 show two types of endovascular catheters; namely passive and active catheters, respectively, during the transendocardial injections of the tested materials, using XMRI hybrid scanner (Figure 11). The active catheter was used for delivering angiogenic genes at the peri-infarct zone in swine and canine hearts subjected to infarction [17,19-21,23-28,30,47-50]. In these studies, the positive effect of the injected genes on myocardial function, perfusion and viability was evident.
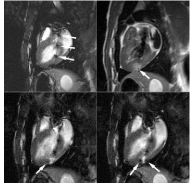
Figure 9: Electrocardiographically gated dual-inversion-recovery T1-
weighted contrast-enhanced MR images (500/40). Top left: The gadolinium
oxide markers (arrows) on the catheter are clearly visualized inside the LV
chamber before the injection of the contrast materials. Top right: Diluted
gadodiamide (0.05 mol/L) produced a bright zone (arrow) by shortening the
myocardial T1. Two injections of undiluted gadodiamide (ie, 0.2 mL of 0.5
mol/L) had been administered to deliver the contrast agent to the borders
of the enhanced area of interest—that is, to hit the target. Bottom left and
right: Undiluted gadodiamide injected into the myocardium created a signal
intensity loss owing to the T2* effect of the contrast material (arrows).
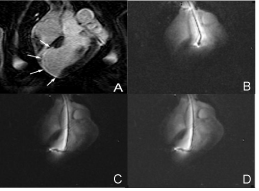
Figure 10: Contrast enhanced MR image shows the scar in the left ventricle
(A, arrows). Real time snapshots of the advancement of the active catheter
into the aorta (B), left ventricle (C) and injection of therapy into the target (D).
This procedure was used to deliver angiogenic genes and labeled stem cells.
MR guided and tagging sequences demonstrated the intramyocardial administration of AAV–VEGF gene and the positive changes in circumferential strain, respectively [23]. Infarct size reduction and the formation of new blood vessels was confirmed on histology (Figure 12). These capabilities have positioned MR imaging as an important approach to pursue for assessing the benefits of delivered genes [18,51,52].
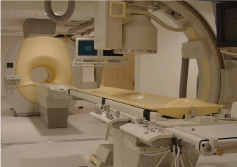
Figure 11: The X-ray/MR suite for interventional radiology at the University
of California San Francisco. The main features of this combined system are a
short-bore magnet, a sliding door between the two systems to shields RF and
X-ray exposure, and a movable tabletop to connect the two systems.
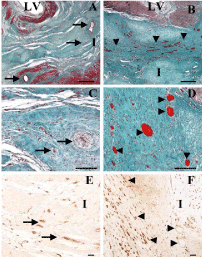
Figure 12: Histology of scar infarct in control (left) and VEGF gene–
treated animals (right) 8 weeks after infarction. Sections A-D were stained
with Masson trichrome stain, while E and F were stained with biotinylated
isolectin B4. I = infarction in both groups which is comprised of homogeneous
replacement fibrosis with a distinct boundary at the interface between scar
and viable Myocardium (M). Treated animal (right) contained numerous
vessels (arrows), while control animal contained very few vessels. Biotinylated
isolectin B4 localized vessels with brown reaction product, accentuating the
neovascularity in VEGF gene–treated infarct animal compared with control
animal. LV = left ventricle, calibration bars = 80 μm.
Future direction: In the future, treatment of ischemic heart disease involves a collaborative effort of molecular/cellular biology, cardiology, pathology and radiology. In this mini-review we report novel approaches for delivering local therapeutic agent in infarcted myocardium and the value of non-invasive MRI in assessing the effectiveness of gene therapies. Future developments in MR-guided procedures include the design of flexible and durable active catheters, which can be tracked and navigated easily. The spatial and temporal resolution of images will also have to be improved to ensure a higher success rate and more accuracy in interventional procedures.
Limitations
The use of endoscopic and endovascular catheter is associated with 10% mortality rate based on our experience. The major complications of in using these devices are LV arrhythmia. In cases of endovascular MR-guided catheter the possibility of perforating the aortic valves and/or myocardium is high as well as the heating in the MRI environment [52]. The limitations of endoscopic approach are pneumothorax and limited access to the septal LV wall.
Conclusion
The endoscopic and endovascular catheter techniques are feasible and minimally invasive. Direct intramyocardial delivery of therapies is useful in occlusive infarct. It provides promising results. Intramyocardial injected genes caused improvement of LV function, regional perfusion and reduction in infarct size measured noninvasively on MRI. The findings confirmed at postmortem histopathologic assessment. Thus, direct delivery of genes in myocardium may be promising in treating patients with ischemic heart disease. Controlled studies with a large population of patients are needed to evaluate the efficacy of direct therapy in promoting myogenesis and angiogenesis. The use of these devices could reduce the pain and speedup the recovery time caused by open-chest surgery. We propose that minimally invasive endoscopic and endovascular catheter techniques and local gene therapies have the potential to change the treatment paradigm in patients with high operative risks, elderly patients, or patients not willing to undergo surgery. Further development of MRI sequences is mandatory for direct intramyocardial drug delivery. It should be noted that at the present time technical challenges remain using both approaches.
References
- Ripa RS, Wang Y, Jorgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006; 27: 1785-1792.
- Liu Y, Rajur K, Tolbert E, Dworkin LD. Endogenous hepatocyte growth factor ameliorates chronic renal injury by activating matrix degradation pathways. Kidney Int. 2000; 58: 2028-2043.
- Lazarous DF, Scheinowitz M, Shou M, Hodge E, Rajanayagam S, Hunsberger S, et al. Effects of chronic systemic administration of basic fibroblast growth factor on collateral development in the canine heart. Circulation. 1995; 91: 145-153.
- Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996; 94: 1074-1082.
- Lazarous DF, Shou M, Stiber JA, Dadhania DM, Thirumurti V, Hodge E, et al. Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution. Cardiovasc Res. 1997; 36: 78-85.
- Lazarous DF, Shou M, Stiber JA, Hodge E, Thirumurti V, Goncalves L, et al. Adenoviral-mediated gene transfer induces sustained pericardial VEGF expression in dogs: effect on myocardial angiogenesis. Cardiovasc Res. 1999; 44: 294-302.
- Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY, et al. Basic fibroblast growth factor in patients with intermittent claudication: results of a phase I trial. J Am Coll Cardiol. 2000; 36: 1239-1244.
- Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005; 112: 1150-1156.
- Fargnoli AS, Mu A, Katz MG, Williams RD, Margulies KB, Weiner DB, et al. Anti-inflammatory loaded poly-lactic glycolic acid nanoparticle formulations to enhance myocardial gene transfer: an in-vitro assessment of a drug/gene combination therapeutic approach for direct injection. J Transl Med. 2014; 12: 171.
- Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA. 1998; 95: 8801-8805.
- Menasche P. Skeletal myoblast transplantation for cardiac repair. Expert Rev Cardiovasc Ther. 2004; 2: 21-28.
- Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001; 357: 279-280.
- Esakof DD, Maysky M, Losordo DW, Vale PR, Lathi K, Pastore JO, et al. Intraoperative multiplane transesophageal echocardiography for guiding direct myocardial gene transfer of vascular endothelial growth factor in patients with refractory angina pectoris. Hum Gene Ther. 1999; 10: 2307- 2314.
- Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized doubleblind placebo-controlled study: the Euroinject One trial. J Am Coll Cardiol. 2005; 45: 982-988.
- Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999; 100: 468-474.
- Saborowski O, Bremerich J, Bongartz G, Saeed M. New therapies for interventional cardiovascular magnetic resonance imaging. Herz. 2008; 33: 323-333.
- Saeed M, Martin A, Jacquier A, Bucknor M, Saloner D, Do L, et al. Permanent coronary artery occlusion: cardiovascular MR imaging is platform for percutaneous transendocardial delivery and assessment of gene therapy in canine model. Radiology. 2008; 249: 560-571.
- Saeed M, Martin A, Ursell P, Do L, Bucknor M, Higgins CB, et al. MR assessment of myocardial perfusion, viability, and function after intramyocardial transfer of VM202, a new plasmid human hepatocyte growth factor in ischemic swine myocardium. Radiology. 2008; 249: 107-118.
- Saeed M, Martin AJ, Lee RJ, Weber O, Revel D, Saloner D, et al. MR guidance of targeted injections into border and core of scarred myocardium in pigs. Radiology. 2006; 240: 419-426.
- Saeed M, Saloner D, Martin A, Do L, Weber O, Ursell PC, et al. Adenoassociated viral vector-encoding vascular endothelial growth factor gene: effect on cardiovascular MR perfusion and infarct resorption measurements in swine. Radiology. 2007; 243: 451-460.
- Saeed M, Lee R, Martin AJ, Weber O, Revel D, Saloner D, et al. MR Guidance of Targeted Injections into Border and Core of Sarred Myocardium. Radiology. 2005.
- Jacquier A, Higgins CB, Martin AJ, Do L, Saloner D, Saeed M. Injection of adeno-associated viral vector encoding vascular endothelial growth factor gene in infarcted swine myocardium: MR measurements of left ventricular function and strain. Radiology. 2007; 245: 196-205.
- Jacquier A, Higgins CB, Saeed M. MR imaging in assessing cardiovascular interventions and myocardial injury. Contrast Media Mol Imaging. 2007; 2: 1-15.
- Jacquier A, Lee R, Martin A, Weber O, Higgins C, Saloner D, et al. editors. Effects of plasmid-VGEF on myocardial infarct: MRI study. ISMRM work shop. 2006.
- Carlsson M, Osman NF, Ursell PC, Martin AJ, Saeed M. Quantitative MR measurements of regional and global left ventricular function and strain after intramyocardial transfer of VM202 into infarcted swine myocardium. Am J Physiol Heart Circ Physiol. 2008; 295: 522-532.
- Dick AJ, Guttman MA, Raman VK, Peters DC, Pessanha BS, Hill JM, et al. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in Swine. Circulation. 2003; 108: 2899-2904.
- Dick AJ, Lederman RJ. MRI-guided myocardial cell therapy. Int J Cardiovasc Intervent. 2005; 7: 165-170.
- Dicks D, Carlsson M, Furtado A, Do L, Saeed M. Rersorption of myocardial edema and microvascular obstrction detected on 64-slice multi-detector computed tomography. 2008.
- Dicks D, Saloner D, Martin A, Ursell P, Carlsson M, Saeed M. Cardiovascular magnetic resonance imaging for percutaneous transendocardial delivery and three dimensional left ventricular strain assessment of VEGF gene therapy in occlusive infarction. Int J Cardiol. 2009.
- Kraitchman DL, Sampath S, Castillo E, Derbyshire JA, Boston RC, Bluemke DA, et al. Quantitative ischemia detection during cardiac magnetic resonance stress testing by use of FastHARP. Circulation. 2003; 107: 2025-2030.
- Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005; 112: 1451-1461.
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation. 2013; 128: 42-49.
- Gyongyosi M, Khorsand A, Zamini S, Sperker W, Strehblow C, Kastrup J, et al. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation. 2005; 112: 157-165.
- Fuster V, Sanz J. Gene therapy and stem cell therapy for cardiovascular diseases today: a model for translational research. Nat Clin Pract Cardiovasc Med. 2007; 4: 1-8.
- San Roman JA, Fernandez-Aviles F. The role of noninvasive imaging techniques in the assessment of stem cell therapy after acute myocardial infarction. Nat Clin Pract Cardiovasc Med. 2006; 3: 38-41.
- Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010; 48: 504-511.
- Bengel FM, George RT, Schuleri KH, Lardo AC, Wollert KC. Image-guided therapies for myocardial repair: concepts and practical implementation. Eur Heart J Cardiovasc Imaging. 2013; 14: 741-751.
- Karmarkar PV, Kraitchman DL, Izbudak I, Hofmann LV, Amado LC, Fritzges D, et al. MR-trackable intramyocardial injection catheter. Magn Reson Med. 2004; 51: 1163-1172.
- Zenati MA, Shalaby A, Eisenman G, Nosbisch J, McGarvey J, Ota T. Epicardial left ventricular mapping using subxiphoid video pericardioscopy. Ann Thorac Surg. 2007; 84: 2106-2107.
- Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005; 96: 151-163.
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004; 364: 141-148.
- Sasano T, Kikuchi K, McDonald AD, Lai S, Donahue JK. Targeted highefficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007; 42: 954-961.
- Katz AM. Proliferative signaling and disease progression in heart failure. Circ J. 2002; 66: 225-231.
- Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, doubleblind, placebo-controlled, phase 2b trial. Lancet. 2016; 387: 1178-1186.
- Schweikert RA, Saliba WI, Tomassoni G, Marrouche NF, Cole CR, Dresing TJ, et al. Percutaneous pericardial instrumentation for endo-epicardial mapping of previously failed ablations. Circulation. 2003; 108: 1329-1335.
- Saeed M, Saloner D, Weber O, Martin A, Henk C, Higgins C, et al. MRI in guiding and assessing intramyocardial therapy. Eur Radiol. 2005; 15: 851- 863.
- Saeed M, Lee R, Martin A, Weber O, Krombach GA, Schalla S, et al. Transendocardial delivery of extracellular myocardial markers by using combination X-ray/MR fluoroscopic guidance: feasibility study in dogs. Radiology. 2004; 231: 689-696.
- Fu Y, Azene N, Ehtiati T, Flammang A, Gilson WD, Gabrielson K, et al. Fused X-ray and MR imaging guidance of intrapericardial delivery of microencapsulated human mesenchymal stem cells in immunocompetent swine. Radiology. 2014; 272: 427-437.
- Dicks DL, Carlsson M, Heiberg E, Martin A, Saloner D, Arheden H, et al. Persistent decline in longitudinal and radial strain after coronary microembolization detected on velocity encoded phase contrast magnetic resonance imaging. J Magn Reson Imaging. 2009; 30: 69-76.
- Saeed M, Henk CB, Weber O, Martin A, Wilson M, Shunk K, et al. Delivery and assessment of endovascular stents to repair aortic coarctation using MR and X-ray imaging. J Magn Reson Imaging. 2006; 24: 371-378.
- Yeung CJ, Susil RC, Atalar E. RF heating due to conductive wires during MRI depends on the phase distribution of the transmit field. Magn Reson Med. 2002; 48: 1096-1098.