
Special Article - Diagnostic Radiology
Austin J Radiol. 2019; 6(2): 1092.
Traumatically-Induced Pseudotumor of Rice Bodies (Corpora oryzoideoma) Around a Prosthetic Hip
Lehmer LM1*, Ragsdale BD2, Miller TL3, Bolivar DA4, Chin G5 and Lamb MC5
1Department of Dermatology, University of California Irvine, USA
2Western Diagnostic Services Laboratory, USA
3Radiology Associates of San Luis Obispo, USA
4Associated Surgeons of San Luis Obispo, USA
5Departmet of Orthopedic Surgery, University of California Irvine, USA
*Corresponding author: Larisa M Lehmer, Department of Dermatology, University of California Irvine, CA 92697, USA/p>
Received: March 11, 2019; Accepted: April 19, 2019; Published: April 26, 2019
Abstract
Although Rice Bodies (RB) most commonly occur as a sequela of chronic rheumatoid arthritis, they may also form in the absence of underlying disease. RBs are grain sized fusiform, or elliptical, concentrically laminated aggregates composed primarily of fibrin. That RBs represent a nonspecific response to synovial inflammation and/or irritation is illustrated by the following case of a 66-year-old male who presented with a massive amalgamation of RBs within the synovial capsule of an artificial left hip after suffering multiple equestrian sportsrelated traumas to the joint. All bacterial cultures were negative. The large size, unusual presentation of RBs as an amalgamated mass versus free-floating particles, and absence of underlying rheumatologic condition motivated further investigation. Histology indicates RBs originate as sterile fibrinous exudate from the denuded synovial/capsular surface and grow by accreting more layers as they tumble. Treatment involves emptying the joint via open incision and addressing the underlying cause.
Keywords: Rice bodies; Trauma; Hip arthroplasty; Fibrin; Pseudotumor
Introduction
Rice Bodies (RBs), named after their macroscopic likeness in color, shape, and size to polished grains of rice, are associated with chronic inflammatory processes, notably rheumatoid arthritis, seronegative inflammatory arthritis, and tuberculous joints [1]. In this report, a massive aggregate of rice bodies around a prosthetic hip was clinically suspected to be a post-traumatic hematoma.
Although RBs can normally be identified on T2-weighted MRI as hypointense fusiform-shaped nodules floating free in a fluid background, in the present case, the absence of a significant liquid milieu obscured RB detection. The rarity of massive RB aggregates, a history of trauma, and its unusual radiologic presentation due to an absence of significant joint fluid to separate the RBs, motivated investigation into the etiology of RB formation in the present case.
Case Report
Thirty-six months prior to presentation, the 66-year-old male patient underwent uncomplicated total left hip replacement for advanced osteoarthritis. Seventy days following left hip replacement, the patient resumed horseback riding 3-5 times a week for a total of 40 hours per month. Sixteen months post-op for left hip arthroplasty he developed moderate, intermittent, idiopathic lateral hip pain with associated tenderness isolated in the trochanteric region and symptoms of trochanteric bursitis. At twenty-eight months post-op, his horse bolted out from under him as he tried to mount and the fall onto his left side led to significant pain and marked swelling about the left knee. The knee improved over the next few days but a newly appreciated area of swelling and marked tenderness appearing over the left greater trochanter
Conventional radiographs of the left pelvis and femur showed no fracture; and confirmed the prosthesis was in good position without loosening. Clinical impression submitted was “severe contusion of trochanteric bursa/greater trochanter with bursitis.”
After three weeks of rest the patient resumed riding. Four months later he sustained a second injury to the same hip from a fall in his yard (7 months after the equestrian accident) and suffered yet a third left hip injury two months after the yard incident whereby the joint was slammed into a metal fence pole during an attempt to dodge a charging heifer. Nine months post-initial injury, physical exam revealed a 10 x 15-cm circumscribed, non-tender soft tissue mass over the rectus femoris. Persistent swelling and enlargement of the left hip was accompanied by pain with both sitting and walking.
MRI displayed an extensive “fluid type mass” deep to the left gluteus maximus that extended both centrally toward the obturator externus and laterally about the greater trochanter and deep to the gluteus medius in addition to regional adenopathy. The mass exhibited a low T1 signal and a high STIR signal which was interpreted as hematoma (Figure 1A&B). Proximity to the trochanteric bursa admits the possibility of bursal rather than articular inception.
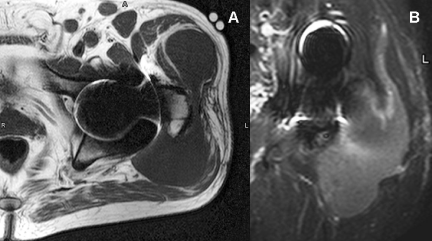
Figure 1: (MRI).
A. Axial T1 (TR 294; TE 17). A large bilobed mass of homogeneously low T1
signal is draped across the greater trochanter.
B. Coronal STIR (TR 3725, TE 60). The same large mass is noted along
the posterolateral aspect of the proximal femur and hip. It exhibits mildly
elevated and heterogeneous T2 signal with a subtle granularity throughout.
Although not recognized at the time of initial interpretation, this pattern no
doubt represents the rice bodies later documented at surgery.
Based on clinical history and imaging, the 12 x 14-cm soft tissue mass over the anterolateral left hip was deemed most likely a hematoma. The unexplained concurrent inguinal and pelvic lymphadenopathy interjected the differential diagnostic possibilities of soft tissue sarcoma, underlying lymphoma, or secondary malignancy. Aspiration at the side of the hip mass produced a proteinaceous coagulation of blood with some neutrophils, and mature collagenous and adipose tissue consistent with mild acute inflammation and no evidence of neoplasm. CT-guided needle biopsy of the wall of left hip collection produced 3 mL of straw colored fluid. Fungal, acid fast bacteria, aerobic and anaerobic bacterial cultures were performed found negative for each biopsy specimen. Six days after needle biopsy, arthrotomy produced a cohesive 14.5 x 8.3 x 3.5-cm mass of white, rice-like material (Figure 2A), which was histopathologically determined to be fusiform fibrin RBs fused together by additional fibrin and a minor amount of clotted blood (Figure 2B) without significant fluid. A 7.5 x 5.3 x 0.5-cm portion of hip joint capsule tissue was additionally removed.
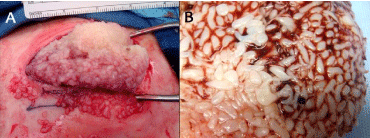
Figure 2: Intraoperative view.
At operation, the mass of fused rice bodies was delivered as a single cohesive
mass (A,B).
Pathologic analysis
RBs consisted of laminated fibrin. Figure 3 depicts a RB situated on the joint capsule from which it originated as a sterile fibrinous exudate. Detached fibrillated portions of fibrous capsule, a minor particulate component in the RBs, had viable connective tissue cells able to survive in the completely avascular intraarticular environment. No detritus from prior operation or from the prosthesis were found in the specimen.
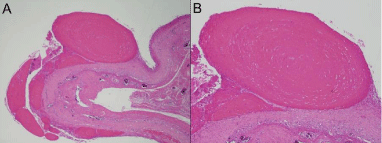
Figure 3: A. An oval fibrin loose body still “in situ,” at least temporarily
attached to the synovial surface (x20).
B. Its concentric lamination, even along the plane of attachment to synovium
(x30), in addition to the size range of less adherent smaller RBs nearby
suggest episodic attachment and liberation into the joint space is the life cycle
of an enlarging rice body.
Follow-up
Except for soreness in the region from the joint replacement and some decreased range of motion, the patient healed without incident. Three months post-op, some swelling returned around the left hip. Joint pain increased and an ultrasound showed a 5-cm collection of fluid lateral and posterior to the greater trochanter not the area of pain. Anteriorly, there was no mass and sterile aspiration indicated no evidence of infection. The patient resumed horseback riding noting some difficulty elevating his foot high enough to engage the stirrup without assisting with his left hand and soreness in the joint when riding referable to the joint replacement rather than the area of mass removal.
Discussion
RBs (also known as corpora oryzoidea - from the Latin for ‘of the body’ corporeus and ‘rice’ oryza) are concentrically laminated masses of fibrin described as fusiform, although they may also be irregularly shaped [2]. The fusiform shape of RBs is the result of mechanical influences particularly the movement of the joint rolling them around which also explains their concentric lamination. RBs most often result from the coagulation of fibrin-forming elements triggered by trauma that damages the endothelium induces fibrin formation in the exudate by activating the platelet cascade [3]. The fusiform shape of fibrinous loose bodies is the result of mechanical influences particularly the movement of the joint rolling them around which also explains their concentric lamination. Because fibrin has high self-affinity [4], pieces of fibrin in the joint capsule stick together as they tumble with the motion of the joint, slowly growing larger as they acquire more layers. Although RBs are well recognized by rheumatologists, they are rarely reported in orthopaedic literature [5].
Traditionally, RBs have been considered a nonspecific response to inflammatory synovial disease and are almost invariably associated with an effusion of fluid into the joint. The most common RB associations are tuberculosis, arthritis deformans, and in Charcot joints. An adult with non-sex-linked congenital hypogammaglobulinemia developed knee swelling with marked synovial proliferation and RB formation without inflammatory cells in synovial fluid and no infiltration of the synovium by lymphocytes or other inflammatory cells [6]. The occurrence of RBs in that patient with non-inflammatory joint disease and the patient here presented underscores the dichotomy of inflammatory reactions in synovitis: cellular infiltration and exudation are two separate processes, the latter not requiring the former.
Multiple RBs in joints or bursae may be the presenting sign of a more extensive underlying rheumatic condition [7]. This patient was not tested for rheumatoid factor or ANA but has no prior history suggestive of inflammatory joint disease. The numerous synovial plasma cells of rheumatoid disease were absent. Synovium and the joint capsule specimen lacked significant inflammation other than a modest, non-granulomatous histiocytic infiltrate. Intraoperative bacterial, fungal, and mycobacterial cultures were negative.
RBs are not peculiar to joints but are also found in tendon sheaths and bursae (i.e. in nonspecific olecranon bursitis8) similarly originating from the synovial membrane.
Cases of RBs containing hydroxyapatite crystals have been reported in patients with ischemic bone necrosis [9]. Macroscopically, the synovial membranes of the affected joints were covered in villous fibrin. Under these circumstances RBs are formed by bone debris from osteonecrotic areas that gained access to the synovial membrane via synovial fluid inducing preferentially “fibrinous inflammation” producing villi with entrapped cartilage and bone fragments of different size that could then detach and form “RBs” with enclosed apatite crystals [10].
RBs are usually present in large numbers ranging from 0.2 to 1.5- cm in length [11], and averaging 0.6-cm in largest dimension. Sugano et al report a case of a “giant” RB 6.5-cm [12]. The consensus is that masses of RBs greater than 10-cm in one dimension are considered “massive.” Since the RB aggregate in this case is 14.5-cm in its largest diameter, it qualifies as massive. Thus far, there have only been two other reports of “massive” RB aggregates: a surgically-removed 30-cm idiopathic mass in an elderly man that had been enlarging over five years to involve the entire pelvic cavity [13] and a 13-cm immobile mass over the volar aspect of the wrist caused by a Mycobacterium marinum infection in a 22-year-old male [14].
The dynamics of RB formation begin with small interstitial, superficial, sub-synovial fibrin deposits that expand to comprise a distinct layer on the joint capsule surface (Figure 3A). Some of this has the concentric laminations of free-floating RBs (Figure 3B), but truncated along the base of attachment, indicating a process of sequential re-teatherings to the surface and partial resorption. The absence of a monolayer of synovial cells beneath the forming RBs (Figure 4) reflects normal micro-anatomy. The synovium, although referred to as a membrane, differs in character from the mesothelium lining the major serous cavities (pleural, pericardial, and peritoneal); the cellular lining of the joint cavity is discontinuous [15]. Strictly speaking, the interior of a synovial joint should be looked upon as a large tissue space rather than as a membrane-lined cavity. Accordingly, in this case, a continuous intimal cell layer is not seen under the fibrin. Shreds of joint capsule plus histiocytes and possibly Type a synovial lining cells (both of which have the capacity to lyse fibrin) become incorporated into the enlarging fibrin aggregate (Figure 4). This is accompanied by focal capillary ingrowth, the body’s attempt at organization of the fibrin. These elements (joint capsule shreds, avulsed capillaries, intimal synovial cells and histiocytes) depart the synovial surface with the fibrin aggregate which becomes a RB free in the joint space. Inert but still cellular tissue shreds nearer the center of a RB (Figure 5A) show the Alcian blue-positivity (Figure 5B) of the joint capsule subintimal layer left behind. It is from the more peripherally situated joint capsule shreds nourished by synovial fluid, that modest collagenization proceeds (Figure 6).
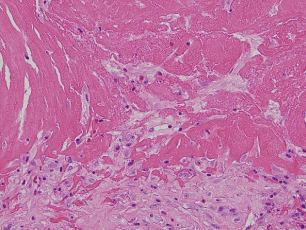
Figure 4: Pennants of the frayed appearing joint capsule without synovial
cell layer extend into the surface accumulation of fibrin, destined to become
minute tissue inclusions when the fibrin globule is exfoliated into the joint
space, loosened by histiocytic proteolysis (x200).
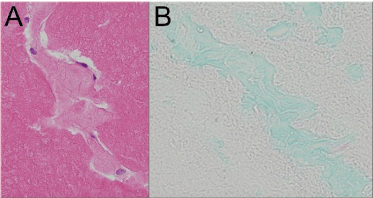
Figure 5: (A) Tissue shreds in central regions of the rice bodies retain
cellularity (H&E, x400) and (B) Alcian blue positivity of normal subsynovium
(Alcian blue, x300).
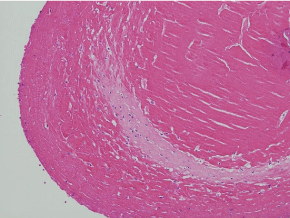
Figure 6: The feathery edge (upper left) of this peripherally located tissue
inclusion suggests collagen synthesis (H&E, x200).
Although RB formation resembles synovial chondromatosis clinically, the literature suggests that analysis of radiographic and MRI appearances should allow discrimination. T2-weighted MR imaging allows diagnosis of RBs due to their “clear delineation from the bursal fluid,” as hypointense nodules relative to surrounding liquid. The typical musculoskeletal hematoma will have an elevated intrinsic T1 signal related to methemoglobin or proteinaceous debris and a heterogeneously bright T2 signal with scattered, usually peripheral, foci of low T2 and T1 signal representing hemosiderin. These imaging features do not characterize the present case. Instead, there was a relatively homogeneous low T1 and high T2 signal. These signal characteristics simply reflect a fluid collection. While a longstanding hematoma was a reasonable point in the radiologic differential diagnosis, given the atypical signal features it is likely the history of trauma influenced the original evaluation.
In retrospect, it is possible to detect a granular pattern within the mass on both T1 and T2 sequences predicting the presence of RBs. In the present case, there was simply too little fluid (i.e. lymphoplasmacytic infiltrate [2], serous fluid [1,12,14], joint effusion [3]) to offset the amalgamated RB signal and the history of trauma likely influenced the original evaluation.
Treatment of RB accumulations is directed towards the cause of the underlying the hydrops. Emptying the joint is best done by open incision as was done in this case (Figure 2).
Conclusion
RB formation should be suspected in patients presenting with a post-traumatic enlarging mass over a prosthetic joint replacement. This case demonstrates the radiologic challenge the faint “granular” texture a mass of amalgamated RBs in a fibrous background displays on T2-weighted MRI in contrast to the ‘classic’ presentation of RBs as hypointense nodules surrounded by fluid.
References
- Kim RS, Lee JY, Jung SR, Lee KY. Tuberculous subdeltoid bursitis with rice bodies. Yonsei Medical Journal. 2002; 43: 539-542
- Chen A, Wong LY, Sheu CY, Chen BF. Distinguishing multiple rice body formation in chronic subacromial-subdeltoid bursitis from synovial chondromatosis. Skeletal Radiol. 2002; 31: 119-121.
- Steinfeld R, Rock MG, Young DA, Cofield RH. Massive subacromial bursitis with rice bodies. Report of three cases, one of which was bilateral. Clin Orthop Relat Res. 1994; 301: 185-190.
- Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964; 145: 1310-1312.
- Grannis GF. Plasma fibrinogen: determination, normal values, physiopathologic shifts, and fluctuations. Clinical Chemistry. 1970; 16: 486- 494.
- Asik M, Eralp L, Cetic O, Altinel L. Rice bodies of synovial origin in the knee joint. Arthroscopy. 2001; 17: E19.
- Taborn JD. Rice bodies in hypogammaglobulinemic arthritis. J Rheumatol. 1981; 8: 165-168.
- Matsumoto T, Fujita K, Fujioka H, Matsushima S, Kuoso K, Yamaguchi S, et al. Massive nonspecific olecranon bursitis with multiple rice bodies. J Shoulder Elbow Surg. 2004; 13: 680-683.
- Li-Yu J, Clayburne GM, Sieck MS, Walker SE, Athreya BH, DeHoratius RJ, et al. Calcium apatite crystals in synovial fluid rice bodies. Ann Rheum Dis. 2002; 61: 387-390.
- Mohr W. On the origin of rice bodies with apatite crystals. Ann Rheum Dis. 2003; 62: 910-912.
- Wynne-Roberts CR, Cassidy JT. Juvenile rheumatoid arthritis with rice bodies: light and electron microscopic studies. Annals of the Rheumatic Diseases. 1979; 38: 8-13.
- Sugano I, Nagao T, Tajima Y, Ishida Y, Ohno T, Nagao K, et al. Variation among giant rice bodies: report of four cases and their clinicopathological features. Skeletal Radiol. 2000; 29: 525-529.
- Tamai O, Mamadi T, Muto Y, Toda T. Large synovial cyst of the pelvis containing rice bodies. A case report. Int Orthop. 1998; 22: 325-327.
- Sanal HT, Zor F, Kocaoglu M, Bulakbasi N. Atypical mycobacterial tenosynovitis and bursitis of the wrist. Diagn Interv Radiol. 2009; 15: 266-268.
- Hettinga DL. Normal joint structures and their reaction to injury. J Orthopaedics and Sports Physical Therapy. 1979; 1: 16-22.