
Case Report
Austin J Radiol. 2019; 6(2): 1095.
Unusual Clinical Presentation and Complications Following Preoperative Embolization of a Large Adrenal Tumour
Garg P1*, Agrwal A1, Bajaj SK1, Misra RN2 and Arora RK2
¹Department of Radiology, Indraprastha University, India
²Department of Surgical Oncology, Indraprastha University, India
*Corresponding author: Garg P, Department of Radiology, Indraprastha University, India, Room no 23, Radiology office, H block, Safdarjung Hospital, New Delhi-110029. India
Received: June 06, 2019;Accepted: July 04, 2019; Published: July 11, 2019
Abstract
Case of young female presenting with secondary amenorrhea, which on further investigation revealed a large intra-abdominal mass, likely arising from adrenal gland. As the tumor was highly vascular with large feeders, she was referred for pre-operative embolisation to reduce the blood loss during surgery. Post embolisation, the patient suffered from an unusual complication of tumoral rupture along with excessive secretion of catecholamines resulting in myocarditis and myocardial infarction. Patient ultimately succumbed to death. Preoperative embolization of a large, hypervascular adrenal mass lesion is not devoid of unusual complications like tumoral rupture and cardiovascular complications even if the tumour is hormonally inactive. This complication is extremely rare and has never been reported in adrenal tumors after embolisation.
Keywords: Pheochromocytoma; Rupture; Embolization; Myocardial Infarction; Catecholamines
Case Report
We present a case of 22 year-old young female presented to the hospital with chief complaints of secondary amenorrhea for the last eight months. Routine gynecological examination, hormonal assays and hysterosalpingography, were within normal limits. Patient also underwent ultrasonography of the abdomen, which revealed a normal uterus and bilateral ovaries; however there was a large mass on the left side of the abdomen situated anterior to the left kidney. The mass was well defined, heterogeneously hypoechoic with multiple high flow vascular channels. CT angiography of the abdomen was done which revealed a large (8 x 8 x 8cm), well encapsulated intra-abdominal mass lying anterior to the left kidney and caudal to the pancreatic tail (Figure 1a and 1b). Position of left kidney was unaltered and left adrenal gland was not separately visualized. Marked tumoral enhancement was seen with central area of necrosis. Multiple intra tumoral vessels were seen. Tumor derived blood supply from ascending branch of the inferior mesenteric artery and lateral branch of the abdominal aorta above the left main renal artery (Figure 1c). Small arterial twigs were also noticed arising from anterior division of left renal artery at hilum. Tumor showed persistent enhancement in the portal venous phase and the venous drainage of the tumor was seen into the left renal vein. In view of these radiologic findings, a diagnosis of hypervascular retroperitoneal mass likely arising from left adrenal gland was made and a tentative diagnosis of pheochromocytoma was given. Patient denied any history of cephalgia, sweating and palpitations. Urinary VMA levels were 40 μmol per 24 hours, serum metanephrine level was 21.7 IU and serum cortisol was 14 IU, which were within normal normal limits. MIBG scanning could not be done due to non-affordability of the patient. Percutaneous biopsy was avoided due to hypervascular nature of the tumor. Patient was referred to interventional radiology unit for preoperative embolization by surgical oncology team.
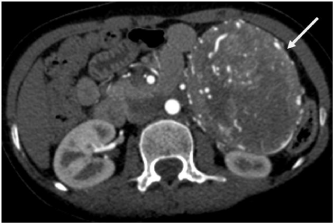
Figure 1a: Axial CT image in arterial phase of contrast enhancement
showing a well defined rounded soft tissue mass lesion on left side lying
anterior to left kidney and showing early enhancement and multiple
intratumoral vessels (solid white arrows).
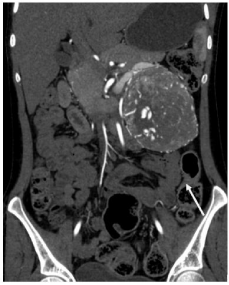
Figure 1b: Coronal image showing the mass lesion with intense vascularity
(straight white arrow) and displacing the pancreas and bowel loops around it.
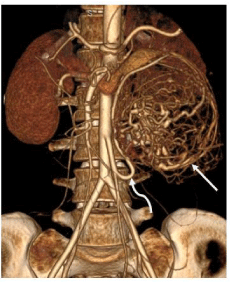
Figure 1c: Volume rendered image showing intense tumoral vascularity
(straight white arrow) and hypertrophied inferior mesenteric artery supplying
the mass (curved white arrow).
After normal preangiography preliminary tests, with anesthesia team ready for the back up, the patient was taken for preoperative embolization in Digital Subtraction Angiography (DSA) lab. Right common femoral access was secured and 5 French renal double curve and Sidewinder catheter were utilized to obtain angiograms from celiac trunk, superior mesenteric artery, inferior mesenteric artery, left renal artery and lateral aortic branches including lumbar arteries. Angiograms revealed major tumor vascularity from the inferior mesenteric artery and direct branch from the aorta likely to be middle adrenal artery (Figure 2a and 2c). Super selective cannulation of these vessels was done using 2.7 French microcatheter [Progreat, Terumo) and embolized with Gelfoam slurry till stasis was observed with markedly decreased tumoral vascularity (Figure 2b and 2d). No arteriovenous shunting was observed within the tumor. Minor arterial supply to the tumor was also observed from the celiac trunk, superior mesenteric artery and left renal artery however these were avoided due to difficulty in superselective cannulation and greater risk of non target embolization. Patient maintained a blood pressure of 110/70 mmHg with SpO2 of 99% in room air throughout the procedure. Patient complained of excessive vomiting and abdominal pain which was expected due to tumoral ischemia which was managed with IV injection of tramadol and ondansatron. Intravenous fluid was started at the rate of 150 mL per hour and patient shifted to high dependency unit in oncology surgery ward after the femoral sheath was removed.
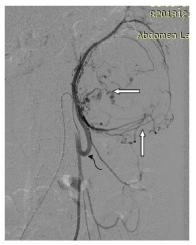
Figure 2a: DSA image showing inferior mesenteric selective angiogram
showing intra and peritumoral hypervascularity (straight white arrows) and
hypertrophied inferior mesenteric artery (curved black arrow).
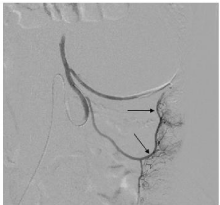
Figure 2b: Post embolization angiogram shows significantly reduced
vascularity and tumoral blush with normal opacification of descending colon
vasa recta (straight black arrows).

Figure 2c: DSA image showing selective angiogram from left middle adrenal
artery (straight white arrow) with tumoral hypervascularity (white star).
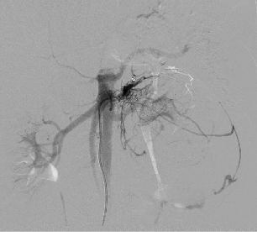
Figure 2d: Post embolization angiogram from middle adrenal artery shows
significantly reduced tumoral vascularity.
During observation in the ward, patient complained of vomiting and persistent abdominal pain with maintained vitals however after a period of 6 hours, patient complained of chest pain and respiratory distress. SpO2 dropped down to 60% in room air and she was intubated emergently and shifted to Intensive Care Unit (ICU). In the ICU, mechanical ventilation was started on CPAP mode and oxygen saturation improved to 85%. On auscultation bilateral basal crepitations were noted and possibility of aspiration pneumonitis, pulmonary edema, pulmonary embolism was considered. Arterial blood gas analysis revealed metabolic acidosis with the base deficit of 10. Patient was stabilized overnight however in the morning she developed hypotension with tachycardia, abdominal distention with decreased bowel sounds and oliguria.
Emergency noncontrast computed tomography scan of the chest and abdomen was done which showed ground glass haziness in bilateral lower lobes with minimal left-sided pleural effusion. Abdominal sections revealed vicarious excretion of the contrast from the distended gallbladder, persistent nephrogram in both kidneys with small peripheral cortical infarcts. The tumor showed internal air foci (likely due to gelfoam embolisation) with hyperdensity suggestive of hemorrhage. Peritumoral, perinephric, retroperitoneal and pelvic hematoma was observed with suggested tumoral rupture (Figure 3a and 3b) and paralytic ileus. Patient also underwent 2-D echocardiography which showed dilated left atrium and ventricle with significant hypokinesia and ejection fraction of 25%. ECG revealed tachycardia and ST segment changes consistent with ischemic pattern. Troponin T was positive suggestive of acute myocardial infarction. Patient was started on inotropic support but patient developed ventricular fibrillation. CPR was started and patient was revived twice but succumbed during the third attempt.
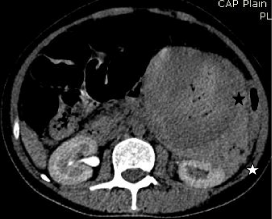
Figure 3a: Non contrast CT axial image showing perinephric hematoma
(white star), intratumoral hemorrhage (black star).
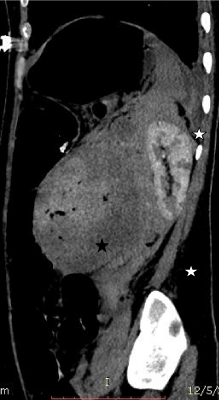
Figure 3b: Saggital CT image showing perinephric (white star) and
intratumoral hemorrhage (black star).
Plausible explanation of this series of events could be tumoral rupture with possible massive release of catecholamines post embolization causing myocardial ischemia and cardiogenic shock with pulmonary venous hypertension and prerenal failure. Patient’s relative did not consent for the autopsy so histopathological diagnosis of the tumor could not be obtained.
Discussion
Pheochromocytoma is a catecholamine secreting tumor from the chromaffin cells of adrenal and extra adrenal sites. The most interesting thing is the variation in its presentation from an incidental finding to classic triad of episodic headache, sweating, and tachycardia.
The diagnosis of pheochromocytoma is dependent on the imaging identification of an appropriately located mass with accompanying clinical and biochemical confirmation. The classic clinical manifestation includes hypertension, which may be episodic or refractory in association with the triad of symptoms of palpitations, headaches, and diaphoresis. The diagnostic evaluation should include measurement of plasma metanephrine levels and of 24-hour urinary catecholamine levels. Indiscriminate biopsy of pheochromocytomas may trigger a catastrophic crisis and must be avoided. Unusual symptoms of secondary amenorrhea and diabetes mellitus due to pheochromocytoma have been reported in the literature by various authors which were controlled by alpha and beta blocker medications [1].
Smaller neoplasms tend to be solid, whereas larger lesions are often hemorrhagic or cystic. Clinically silent pheochromocytomas also tend to be larger [2]. Other reported pathologic features include necrosis, calcification, and fibrosis. These gross features of pheochromocytomas have correlative radiologic features. Pheochromocytomas typically enhance avidly but can be heterogeneous or show regions of no enhancement due to cystic changes. When early intense enhancement occurs, it reflects the capillary-rich framework of the tumor. Pheochromocytomas, in typical inconsistent fashion can demonstrate different and variable washout patterns and may again, therefore be confused with either adenomas or metastases .
PPreoperative adrenal tumor embolization has been attempted by many interventional radiologists in the past. It has known to cause reduction in the size of the tumor, reducing vascularity before surgery, suppression of the hormonal axis and renal hormone production and in particular circumstances to maintain hemostasis in the eventuality of spontaneous tumoral rupture.Prabhasvat et al published a case report where they have presented the patient’s of adrenal tumor embolization each of adrenal carcinoma, cavernous hemangioma and myelolipoma and this technique resulted in successful embolization with decrease in tumoral vascularity and pain in all the patients. No mortality was observed however two of the three patients had postembolization syndrome. In one of the patient, they observed tumoral rupture and hemorrhage after embolization which was controlled with blood transfusion. They did not notice any tumour volume shrinkage in the follow-up [3].
Adrenal gland has three arterial supply from superior middle and inferior adrenal arteries which are the branches of inferior phrenic, aorta and renal arteries respectively [4]. In the large adrenal tumor arterial supply outside the renal artery can be seen as in our case, since a highly vascular mass can parasitize additional arterial supply from neighbouring areas.
Spontaneous rupture of a pheochromocytoma is a rare complication and tumoral rupture postembolization is even rarer. Massive release of catecholamines as well as blood loss can be lifethreatening for the patient. Pua et al. have reported one such case where they had embolized a ruptured pheochromocytoma with PVA particles to control bleeding. Their angiograms also demonstrated peripheral tumoral blush and absent central vascularity as in our case [5].
Many researchers have also embolized pheochromocytoma using Gelfoam with decreased requirements of antihypertensive drugs after the procedure [6,7].
Transarterial embolization of pheochromocytoma has been done previously to manage hypertensive crisis by Hrabovsky et al. [7]. Many researchers have shown the efficacy of transarterial embolization of both adrenal and extra-adrenal paragangliomas [8]. Daniele et al had embolized a large pelvic sympathetic paraganglioma in a young female by cannulating the inferior mesenteric artery [9].
Classic clinical triad of pheochromocytoma rupture is intense vasoconstriction, tachycardia and labile hypertension [10] however this is demonstrated in less than one third of the patient’s having tumoral rupture. Other common clinical features reported are loin pain, nausea and vomiting [11].
Amongst adrenal masses, pheochromocytoma is the most common tumor to bleed. Approximately 50% of adrenal hemorrhagic masses were due to pheochromocytoma in a study by Marti et al. [12]. Risk factors for pheochromocytoma rupture are trauma, anticoagulation and initiation of alpha blocker therapy [13]. Another mechanism could be tumoral necrosis induced cell destruction with massive release of catecholamines causing vasoconstriction, elevated intracapsular pressure resulting in tumoral rupture and hemorrhage [14].
Many authors have authenticated the fact of catecholamineinduced cardiogenic shock. Acute myocardial infarction can be due to hyperdynamic circulation causing increased myocardial oxygen demand or direct toxicity of catecholamines to myocardial cells. Cardiovascular complications like myocarditis, myocardial infarction and heart failure is likely due to massive dumping of catecholamines especially when vascular supply to the tumor has been blocked [15].
Conclusion
In our index case, the imaging features were that of pheochromocytoma however, unusual clinical symptoms like amenorrhea and lack of hypertensive symptoms like diaphoresis and headache along with negative urinary and serum catecholamine markers was a clinical enigma for us and after reviewing the literature of preoperative embolization of adrenal tumors and pheochromocytomas, the decision of embolization was done. Unusual complication of tumoral rupture with possible catecholamineinduced myocardial infarction and cardiogenic shock occurred which were also unusual and unexpected complications. With the help of this article, this can be learning experience that adrenal masses should be handled with caution and any endovascular embolization therapy is not free of the risks and complications that can occur even after the procedure.
References
- Wakil A, Atkin S. Secondary amenorrhoea due to pheochromocytoma: a case report. Cases J. 2008; 1: 30.
- Ramsay JA, Asa SL, van Nostrand AW, Hassaram ST, de Harven EP. Lipid degeneration in pheochromocytomas mimicking adrenal cortical tumors. Am J Surg Pathol. 1987; 11: 480-486.
- Prabhasavat K, Ruamcharoenkiat S. Outcomes of Arterial Embolization of Adrenal Tumor in Siriraj Hospital: Case Report. J Med Assoc Thai. 2015; 98: 621-627.
- Merklin RJ, Michels NA. The variant renal and suprarenal blood supply with data on the inferior phrenic, ureteral and gonadal arteries: a statistical analysis based on 185 dissections and review of the literature. J Int Coll Surg. 1958; 29: 41-76.
- Pua U, Wong DE. Transarterial embolisation of spontaneous adrenal pheochromocytoma rupture using polyvinyl alcohol particles. Singapore Med J. 2008; 49: e126–e130.
- Bunuan HD, Alltree M, Merendino KA. Gel foam- embolization of a functioning pheochromocytoma. Am J Surg. 1978; 136: 395–398.
- Hrabovsky EE, McLellan D, Horton JA, Klingberg WG. Catheter embolization: preparation of patient with pheochromocytoma. J Pediatr Surg. 1982; 17: 849-850.
- Ali AM, Devbhandari M, Sastry A, Ashleigh RJ, Jones MT. Preoperative embolization followed by surgical excision of an intrapericardial pheochromocytoma. Annals of Thoracic Surgery. 2007; 83: 302–304.
- Di Daniele N, Canale MP, Tesauro M, Rovella V, Gandini R, Schillaci O, et al. Preoperative embolization reduces the risk of catecholamines release at the time of surgical excision of large pelvic extra-adrenal sympathetic paraganglioma. Case Rep Endocrinol. 2012; 481328.
- Forty J, Dale RF. Ruptured phaeochromocytoma: a case report. J Royal College of Surg Edinb.1989; 34: 109-110.
- Chan MK, Tse HW, Mok FP. Ruptured phaeochromocytoma—a lesson in acute abdomen. Hong Kong Med J. 2003; 9: 221-223.
- Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012; 36: 75–82.
- Van Way CW, Faraci RP, Cleveland HC, Foster JF, Scott HW Jr. Hemorrhagic necrosis of pheochromocytoma associated with phentolamine administration. Ann Surg. 1976; 184: 26–30.
- Habib M, Tarazi I, Batta M. Arterial embolization for ruptured adrenal pheochromocytoma. Curr Oncol. 2010; 17: 65–70.
- Charon P, Hamwi A, Laigneau P, Beroud P. Forme pseudo-coronarienne de rupture h´emorragique d’un pheochromocytome -A propos d’une observation. Annales De Cardiologie Et D’Angeiologie. 1991; 40: 193–197.