
Special Article - Brain Tumor
Austin J Radiol. 2019; 6(3): 1101.
Ventricular Irruption Using 5-Aminolevulinic Acid in Patients with Glioblastoma
Salvador Manrique-Guzman* and Alejandro González-Garay
¹Department of Neurosurgery, Hospital San Ángel Inn Patriotismo, Mexico City, Mexico
²Methodology Research Unit, Instituto Nacional de Pediatría, Mexico City, Mexico
*Corresponding author: Salvador Manrique-Guzman, Hospital San Ángel Inn Patriotismo, Avenida Patriotismo 67. Office 721. Col. San Juan, Mexico City, Mexico
Received: October 09, 2019; Accepted: October 21, 2019; Published: October 28, 2019
Abstract
Glioblastoma is one of the most malignant brain tumors with a mean survival time in adults of 12-16 months after diagnosis. New evidence points toward always achieving maximal safe surgical resection. Tumor located near to the lateral ventricles can have increased risk of ventricular breach. Ventricular irruption during surgery increase the possibility of acquiring hydrocephalus. We prospectively reviewed 168 patients with newly diagnosed and previously untreated GBM diagnosed between 2005 and 2013 at a single center. Ventricular irruption was register if stated in the surgical technique note or when evident in the postoperative scan. A total of 48 patients were recruited, 5-ALA guided surgery was employed in 30 cases of total resection, 17 for subtotal resection and 1 biopsy. There was not increased risk of ventricular irruption during 5-ALA surgery. Data presented in this study suggest that 5-ALA guided surgery does not increases the risk of ventricular irruption and neither the development of late-onset communicating hydrocephalus.
Keywords: High-grade glioma; Aminolevulinic acid; Lateral ventricles; 5-ALA surgery
Introduction
Glioblastoma is one of the most malignant brain tumors with a mean survival time in adults of 12-16 months after diagnosis. New evidence points toward always achieving maximal surgical resection. Surgery provides some survival benefit (›78% resection), rapid reduction of tumor bulk mass effect with concomitant symptoms palliation and provides tissue for histopathological diagnosis [1-3]. Despite major advances in microsurgical techniques and technological nuances, the median survival still less than 15 months [4,5]. Tumor located near to the lateral ventricles can have increased risk of ventricular breach.
Ventricular irruption during surgery increase the possibility of acquiring hydrocephalus, estimated to occur in 15% of surgical cases with a 4% incidence [6-8]. A ventricular entry during resection may be associated with CFS dissemination of the tumor cells, and CFS dissemination leads to CFS malabsorption followed by postoperative communicating hydrocephalus [9].
The 5-Aminolevulinic Acid (5-ALA) fluorescence guided resection for high-grade glioma has become a useful took to achieve maximal safe tumor removal decreasing the probability of local recurrence [10]. The range of tumor resection can be enhanced under fluorescence guidance with Protoporfirin IX (PpIX) synthesized by 5-ALA. With the intention of achieving a gross total resection for the treatment of glioblastoma, the incidence of ventricular entry during resection may increase, especially tumors located near the lateral ventricles are more prone to by breached during tumor resection [9]. The aim of this paper is to establish the relationship between ventricular irruption and use of 5-ALA fluorescence guided surgery in high-grade tumors.
Methods
We prospectively reviewed 168 patients with newly diagnosed and previously untreated GBM diagnosed between 2005 and 2013 at a single center in University of Tübingen Hospital, Germany, who had ventricular irruption during tumor resection using 5-ALA [11,12]. We included all adult patients had high-grade glioma cytoreductive surgery using 5-ALA for fluorescence guided resection, the exclusion criteria like surgery rejection, known 5-ALA allergy, coagulopathy, absence of postoperative imaging (‹72 hrs) and porphyria, but no cases were register Perioperatively, all patients received 5-ALA 3 hours prior to surgery in a dose of 20 mg per kilogram body weight. Intraopratively, fluorescence was visualized using an adapted microscope (Pentero, Carl Zeiss Meditec, Oberkochen, Germany). Biopsy samples were included. Radiotherapy and concurrent Temozolamide (TMZ) were given according to standard guidelines (75mg/m2 per day) [4]. Ventricular irruption was register if stated in the surgical technique note or when evident in the postoperative scan Figure 1. Epidemiological data (age, gender), data regarding tumor localization and further progression were collected if available. We divided the 5-ALA patient according to the risk of ventricular irruption in relationship to it’s location near the lateral ventricles. Since this was a retrospective review using patients electronic chart, no patient consent or ethical committee was obtained.
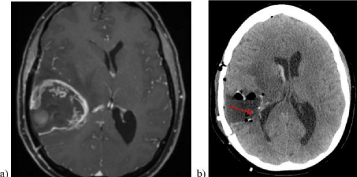
Figure 1: Case illustration: a) Pre-operative contrast enhanced T1 MRI of a
43-old female with a right temporal GBM. The ipsilateral ventricle is deviated
to the contralateral side due to tumor volume and edema. B) Post-operative
CT with evident ventricular breach (red arrow).
Clinical data
Clinical data was collected retrospectively from electronic chart of patients treated by chemo radiotherapy within the study period (n=168). Age, sex, Karnofsky Performance Score (KPS) at the time of diagnosis and after surgery, tumor location, preoperative and postoperative tumor volume (Brainlab Software. Brainlab AG, Feldkirchen, Germany) and dates of death or last register visit were recorded. The extent of resection was determined based on a postoperative MRI performed ‹72 hours after surgery. Gross total resection was defined as no residual tumor enhancement on MRI, while subtotal resection was defined as residual nodular enhancement on MRI. The postoperative MRI scan was acquired in the first 72 hours following the surgery.
Statistical analysis
Categorical data are presented as frequencies and corresponding percentages, while continuous data are presented as median and range. To analyze tumor volume, we used the Kruskall Wallis test. Time-to-event distributions were estimated using the Kaplan-Meier method and compared with the log-rank test. The two groups were stratified according to a higher risk of irruption versus low risk while using fluorescence-guided surgery. A p‹0.05 will be considered statistically significant. Analysis was conducted using STATA 14.1
Results
We collected a total of 168 candidates harboring high-grade glioma; we categorized them according to extent of resection into total (n=91), subtotal (n=53) and biopsy (n=24). A total of 48 patients were recruited, 5-ALA guided surgery was employed in 30 cases of total resection, 17 for subtotal resection and 1 biopsy (p=0.001). There was no significant difference in age with a mean range of 59 years old (32-73). Age at diagnosis was not statistically significant with a mean age of 57 (30-71). Karnofsky Performance Status was not different before and after the surgery in any group with preoperative score of 90 (50-100) and postoperative score of 85 (50-100) (Table 1).
Characteristics
Total Resection
n = 30
Partial Resection
n = 17
Biopsy
n = 1
p
Median (min - max)
Median (min - max)
Median (min - max)
Age (years)
59 (32 – 71)
53 (32 – 73)
62 (-)
0.45
Age at diagnosis (years)
57 (31 – 71)
52 (30 – 71)
60 (-)
0.60
Preoperative Karnofsky
90 (50 – 100)
90 (60 – 100)
70 (-)
0.20
Postoperative Karnofsky
90 (50 – 100)
80 (50 – 100)
80 (-)
0.78
Preoperative volume (cm3)
26.39 (1.03 – 87.8)
40.65 (2.2 – 88.5)
32.7 (-)
0.41
Postoperative volume (cm3)
0.5 (0 – 42.7)
3.6 (0 – 88.5)
4 (-)
0.19
Frequency (%)
Frequency (%)
Frequency (%)
Male
19 (0.63)
11 (0.64)
0 (-)
0.50
Female
11 (0.36)
6 (0.35)
1 (1.0)
0.50
Death
20 (0.66)
13 (0.76)
1 (1.0)
0.66
Intraoperative tools
Neurological monitoring
1 (0.03)
1 (0.05)
1 (1.0)
0.063
IRM
1 (0.03)
1 (0.05)
0 (-)
1.00
Navigation
11 (0.36)
4 (0.23)
0 (-)
0.668
USG
8 (0.26)
4 (0.23)
0 (-)
1.00
No other
9 (0.3)
7 (0.41)
0 (-)
0.68
Tumor Location
Frontal
6 (0.20)
8 (0.47)
1 (1.0)
0.048*
Parietal
8 (0.26)
4 (0.23)
0 (-)
1.00
Temporo-insular
15 (0.50)
11 (0.64)
0 (-)
0.36
Occipital
5 (0.16)
1 (0.05)
0 (-)
0.47
Diencephalic
1 (0.03)
2 (0.11)
0 (-)
0.57
Cerebellar
1 (0.03)
0 (-)
0 (-)
1.00
Table 1: Demographical characteristics.
There were no differences among sex distribution between male and female. Concomitant use of other intraoperative tools was no significant (Table 2). Death risk was not higher within any group. The most frequent tumor location for total resection was temporoinsular and frontal lobe for partial resection (including biopsy) (Table 3). Neurological deficit was the most common postoperative complication, followed by anopsia. There was not increased risk of ventricular irruption during 5-ALA surgery (P=0.52). Late-onset hydrocephalus was not higher in 5-ALA guided surgery (P=0.20). Survival did not differ within the two groups (Log-Rank=0.082). To assess the risk of ventricular irruption we subdivided the population into high-risk and low-risk according to the tumor localization near the lateral ventricles (Table 2).
Characteristics
High risk of ventricular irruption
N = 35
Low risk of ventricular irruption
N = 13
p
Frequency (%)
Frequency (%)
Resection
Total
21 (0.6)
9 (0.69)
0.74
Partial
13 (0.37)
4 (0.30)
0.74
Ventricular irruption
12 (0.34)
8 (0.61)
0.11
Late-onset hydrocephalus
1 (0.02)
3 (0.23)
0.055*
Neurological deficit
7 (0.20)
2 (0.15)
1.00
Death
23 (0.65)
11 (0.84)
0.29
Statistical test = Fisher’s exact test *p = 0.05
Table 2: Postoperative Complications according to the risk of ventricular irruption.
Discussion
Surgical resection of high-grade glioma has evolved in the last decade in achieving maximal safe resection and most prospective data indicates a trend without level I evidence that points extent in survival. Different technologies have aid to pursue that goal like neurological monitoring, Neuronavigation systems, etc. Stummer et al. has extensively work in developing and bang the usefulness of 5-ALA guided surgery [13-15]. To our knowledge, no other research has been conducted to assess the additional risk of ventricular irruption using 5-ALA guided surgery.
Ependymal cells in the lateral ventricles has been pointed to possess a highly autofluorescence capacity, also reported by Hayashi as fluorescence of the ventricular wall but no tumor cell invasion macroscopically [9]. Autofluorescence, results as the emission spectra of a fluorophores of porphyrin chemical structures, but it will be only be visible exposing the ependymal cells through ventricular opening [16]. In our population, ventricular irruption while using 5-ALA was not an independent risk of late-onset hydrocephalus. In some cases, surgeons attempt to “chase” fluorescence under the microscope Figure 2.
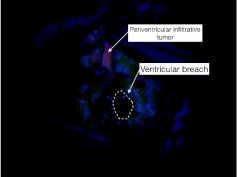
Figure 2: Intraoperative photograph using an adapter microscope (Pentero,
Carl Zeiss Meditec, Oberkochen, Germany) for 5-ALA (20mg/kg body-weight
3 hours prior to surgery) with solid fluorescence tumor near the right lateral
ventricle after ventricular breach and vague fluorescence in the nearby
ependyma.
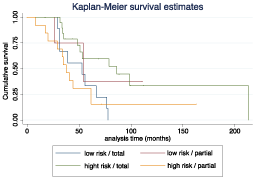
Figure 3: Survival analysis comparing the low/high risk of ventricular irruption.
Survival did not differ within the two groups.
Ventricular breach can occur most frequent in tumors located near the lateral ventricles, the survival graph of our population showed partial resection decreases survival and that can be explained as consequence of tumor cytoreduction. In our population we did not find a direct effect of 5-ALA surgery and increased ventricular irruption (OR=1.26 (0.63–2.50) p=0.50) and neither in the development of late-onset hydrocephalus (OR=0.92 (0.27–3.05) p=0.89). A recent retrospective study by Behling et al. confirmed in a 229-patient recruitment that ventricular opening is safe and should be consider to achieve maximal tumor resection [17].
Conclusion
Data presented in this study suggest that 5-ALA guided surgery does not increases the risk of ventricular irruption and neither the development of late-onset communicating hydrocephalus. Surgeon must always remember the anatomical landmarks when performing tumor resection and avoid following the inertia of “chasing fluorescence”. 5-ALA guided surgery can be useful in tracking ependymal and subependymal spread.
Disclousure
The authors report no conflict of interest concerning the materials or methods used in this study of the finding specified in this paper. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sector. Dr. Salvador Manrique-Guzman received a grant form the WFNS-Aesculap Fellowship.
Acknowledgment
I want to thank Professor Marcos S. Tatagiba for allowing me to pursuit the publication of this manuscript.
References
- Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir (Wien). 2011; 153: 1211-1218.
- Bloch O, Han SJ, Cha S, Sun MZ, Aghi MK, McDermott MW, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg. 2012; 117: 1032-1038.
- Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999; 52: 371-379.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352: 987-996.
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008; 359: 492-507.
- Marquardt G, Setzer M, Lang J, Seifert V. Delayed hydrocephalus after resection of supratentorial malignant gliomas. Acta Neurochir (Wien). 2002; 144: 227-231.
- Bulstrode HJ, Natalwala A, Grundy PL. Atypical presentation of delayed communicating hydrocephalus after supratentorial glioma resection with opening of the ventricles. Br J Neurosurg. 2012; 26: 222-226.
- Fischer CM, Neidert MC, Peus D, Ulrich NH, Regli L, Krayenbuhl N, et al. Hydrocephalus after resection and adjuvant radiochemotherapy in patients with glioblastoma. Clin Neurol Neurosurg. 2014; 120: 27-31.
- Hayashi Y, Nakada M, Tanaka S, Uchiyama N, Hayashi Y, Kita D, et al. Implication of 5-aminolevulinic acid fluorescence of the ventricular wall for postoperative communicating hydrocephalus associated with cerebrospinal fluid dissemination in patients with glioblastoma multiforme: a report of 7 cases. J Neurosurg. 2010; 112: 1015-1019.
- Kamp MA, Felsberg J, Sadat H, Kuzibaev J, Steiger HJ, Rapp M, et al. 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir (Wien). 2015; 157: 207-213.
- Tejada-Solis S, Aldave-Orzaiz G, Pay-Valverde E, Marigil-Sanchez M, Idoate-Gastearena MA, Diez-Valle R. Prognostic value of ventricular wall fluorescence during 5-aminolevulinic-guided surgery for glioblastoma. Acta Neurochir (Wien). 2012; 154: 1997-2002.
- Leonhard M. New incoherent autofluorescence/fluorescence system for early detection of lung cancer. Diagn Ther Endosc. 1999; 5: 71-75.
- Tonn JC, Stummer W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: practical use, risks, and pitfalls. Clin Neurosurg. 2008; 55: 20-26.
- Stummer W. Response to journal club: 5-aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2015; 76: 230-231.
- Ewelt C, Nemes A, Senner V, Wolfer J, Brokinkel B, Stummer W, et al. Fluorescence in neurosurgery: Its diagnostic and therapeutic use. Review of the literature. J Photochem Photobiol B. 2015; 148: 302-309.
- Croce AC, Bottiroli G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur J Histochem. 2014; 58: 2461.
- Behling F, Kaltenstadler M, Noell S, Schittenhelm J, Bender B, Eckert F, et al. The Prognostic Impact of Ventricular Opening in Glioblastoma Surgery: A Retrospective Single Center Analysis. World Neurosurg. 2017; 106: 615-624.