
Special Article - Nutrition Diet
Int J Nutr Sci. 2019; 4(2): 1033.
Citrulline: Modulation on Protein Synthesis, Intestinal Homeostasis and Antioxidant Status
Xu XM1,2, Wu CM1,2, Jia G1,2, Zhao H1,2, Chen XL1,2, Wang J3 and Liu GM1,2*
1Institute of Animal Nutrition, Sichuan Agricultural University, China
2Key Laboratory for Animal Disease-Resistance Nutrition of China Ministry of Education, Sichuan Agricultural University, China
3Maize Research Institute, Sichuan Agricultural University, China
*Corresponding author: Liu GM, Institute of Animal Nutrition, Sichuan Agricultural University, 211 Huimin St, Wenjiang District, Chengdu, Sichuan, China, Email: liugm@sicau.edu.cn
Received: June 17, 2019;Accepted: July 09, 2019; Published: July 16, 2019
Abstract
Citrulline, a nonessential amino acid, is an important precursor substance of arginine. It has been implicated in diverse biological and physiological events, such as nitrogen balance, growth and development, muscle performance, and intestinal homeostasis, but its underlying mechanism in intestinal function is unclear. This review discusses the modulation effects of citrulline on protein synthesis and intestinal homeostasis, as well as its potential mechanisms to broaden its application.
Keywords: Citrulline; Protein synthesis; Intestinal homeostasis; Antioxidant; Nitrogen balance; Gut barrier
Introduction
Citrulline (C6H13N3O3), a key intermediate of urea cycle, mainly exist in the watermelon in nature [1]. In mammals, enterocytes have a significant effect on the circulating citrulline levels [2]. Citrulline is essential for various regulation processes, especially during sepsis and endotoxemia [3], and plays an important role in protein synthesis and intestinal homeostasis. Studies have found that citrulline regulates nitrogen balance [4], growth and development [5,6], muscle performance [7], gut barrier function [8-11] intestinal digestion and absorption [12], intestinal cell apoptosis [13] and antioxidant function [4,14,15]. The present review briefly highlights the significant role of citrulline in animals in the modulation of protein synthesis and intestinal homeostasis and points out its potential mechanisms.
Physicochemical property
The term citrulline (Figure 1) was derived from watermelon (Citrullus vulgaris) in the 1930s [16]. Citrulline has a molecular weight of 175.19 g/mol and presents two enantiomers, namely, the L and D forms. In nature, citrulline exists in the L form. Under normal temperature and pressure, citrulline is a white crystal or crystalline powder. Citrulline has a melting point of 222 °C and density of 1.289 g/cm [17]. It is well soluble in water but not in ethanol, ether, and methanol [17].
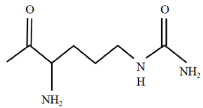
Figure 1: Citrulline structure [16].
Biosynthesis and Metabolism
Citrulline is synthesized from substrate of arginine, proline, glutamine in the intestine (Figure 2). The common metabolic product of arginine, proline, and glutamine is ornithine, which is then converted to citrulline. The citrulline synthesized by glutamine accounts for the major proportion of the intestinal epithelial cell circulating citrulline levels [18]. The conversion of glutamine to citrulline depends on four enzymes: glutaminase metabolizes glutamine to glutamate, and glutamate regulates the synthesis of pyrroline through Pyrroline-5-Carboxylate Synthase (P5CS). Then, Ornithine Aminotransferase (OAT) converts P5C to ornithine, and Ornithine Carbamoyltransferase (OCT) converts ornithine to citrulline. Arginine converted to n-hydroxy-arginine by the catalysis of Nitric Oxide Synthase (NOS). N-Hydroxy-arginine is then converted to citrulline, and the process releases NO [2,16,18]. Furthermore, arginine can be converted into ornithine by the action of arginase and then into citrulline. Arginase is a key rate-limiting enzyme in the transformation of arginine into ornithine. Proline is metabolized to P5C at first under the action of proline oxidase, then metabolized to ornithine under the action of OAT in the small intestine, liver and kidneys [18]. CO2 and NH3 are transferred to citrulline by N-acetylglutamate synthase and Carbamoyl Phosphate Synthase I (CPS-I). The intermediate metabolites are N-acetylglutamate and carbamoyl phosphate [2]. Additionally, in the kidney, citrulline transferred to arginine by ASS and ASL contributes to the majority of endogenous arginine levels [2,17,19]. Citrulline metabolism involves three different pathways: arginine biosynthesis, NO cycle, and complete urea cycle, which take place in the whole body, local sites, liver and kidneys, respectively [16].
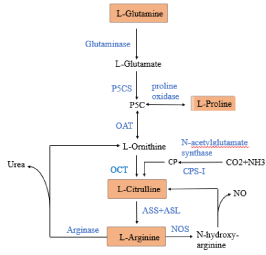
Figure 2: Scheme of synthesis of L-citrulline [2]. P5C: l-Δ1-Pyrroline-5-
Carboxylate; P5CS: Pyrroline-5-Carboxylate Synthase; OAT: Ornithine
Aminotransferase; OCT: Ornithine Carbamoyltransferase; ASS:
Argininosuccinate Synthetase; ASL: Argininosuccinate Lyase; CP:
Carbamoyl Phosphate; CPS-I: Carbamoyl-Phosphate Synthase I; NOS: Nitric
Oxide Synthase.
Modulation Effects of Citrulline on Protein Synthesis
Citrulline and nitrogen balance
Nitrogen balance is the balance between nitrogen intake and output. It exerts its regulation effects by affecting urea formation. The arginine/glutamine–citrulline cycle contributes to ureagenesis. The liver can absorb arginine and glutamine, activate ureagenesis, and consequently increase amino acid metabolism and protein synthesis. Citrulline synthesis from arginine and glutamine prevents the overactivation of urea formation, thus maintaining the nitrogen balance.
Citrulline is crucial for keeping nitrogen homeostasis. In the absence of arginine supplementation, the concentration of plasma arginine does not decrease, and the plasma citrulline concentration is increased [20]. Citrulline treatment, but not arginine supplementation, increases the concentration of plasma arginine in horses [21] and restores the production of NO in small intestinal cells and the nitrogen balance [3,22-25]. Citrulline supplementation improves the activity and expression of kidney arginine succinate synthase and arginine concentration [12]. This arginine–citrulline cycle is a mean to protect dietary arginine from excessive liver degradation. Moreover, in a mouse model fed with an arginine-free diet, ornithine contributes to citrulline synthesis [26] and ornithine synthesis from citrulline in the small intestinal mucosa of mice [27]. After citrulline is synthesized from ornithine by enterocytes, it is then largely absorbed by the kidneys [28]. In the kidney, almost all the citrulline is metabolized to produce arginine under the action of argininosuccinate synthetase and argininosuccinate lyase. In conclusion, the arginine–ornithine– citrulline cycle is central to maintaining nitrogen homeostasis.
Citrulline and growth
Animal growth and development is the guarantee of efficient production, which is influenced by many factors, such as animal species, nutrition level, environment and maternal effect [29-31]. Citrulline supplementation is an important nutrition regulator in animal growth and development. In the cases of intrauterine growth restriction, the supply of citrulline promotes fetal development. Fetal growth enhancement is related to increased fetal muscle protein synthesis [5,6]. This promotion effect may also be related to increased hormone secretion. Citrulline contributes to the release of a variety of endocrine hormones, mainly including the growth hormone, insulin [32]. Furthermore, these hormones are contributed to protein synthesis [33-36]. These findings are in agreement with those of previous studies, which found that arginine and its metabolites are responsible for the secretion of insulin and growth hormone and influence piglet growth [18,37,38]. When mice fed with an arginine-free diet are weaned, their citrulline production increases, and the mice achieve normal weight [39]. Furthermore, citrulline administration increases the body weight and average daily gain in suckling piglets, and the suitable adding dose is 0.29 g/kg/day [12]. However, the precise mechanisms behind these processes remain unclear.
Citrulline and muscle performance
Animal muscle performance is related to the supply of certain nutrients. It’s reported that citrulline could be transported to rat aortic smooth muscle cells by a low-affinity carrier with characteristics resembling systems L and N [40]. Citrulline exerts its promotion effects on muscle performance by enhancing muscle protein synthesis and protecting the muscle cells from atrophy [14,41-44]. The supply of citrulline increases the synthesis rate of muscle protein by 33% in male rats [45]. Citrulline affects muscle protein synthesis by the following action: increasing myofibrillar protein synthesis [41]; enhancing the expression of skeletal muscle myofibrillar constituents [14]; improving insulin levels [42]; and increasing muscle mass, content, strength, and motor activity[46]. The positive effect of citrulline on muscles is related to three mechanisms. First, the implication of the NO pathway. NO synthesis depends on NOS, which includes nNOS, eNOS, and iNOS [47]. L-citrulline supplementation significantly increases protein synthesis rate and the diameter of C2C12 myotubes incubated in HBS or serum free media and in the presence of NOS inhibitors (NG-nitro-L-arginine methyl ester (L-NAME) and aminoguanidine). L-citrulline significantly increases iNOS gene expression [48]. In one study, male Wistar rats were assigned to four groups: recovered, 1-(2-Trifluoromethylphenyl)- Imidazole (TRIM), L-NAME, and control. The TRIM is also an inhibitor of NOS. The cross-sectional area of skeletal muscle in the recovered group reached complete muscle regrowth which exceeds the l-NAME and TRIM group after 10 days of immobilization and 7 days of recovery [49]. These results show that the NOS pathway is vital for the regrowth of immobilized muscles. Moreover, the activation of the mTOR pathway is involved in the muscle regulation process. mTOR expression in the recovery group of male Wistar rats was enhanced compared to the control group and the increase was blocked in the l-NAME and TRIM groups after 10 days of immobilization and 7 days of recovery [49]. Moreover, citrulline activates the phosphorylation of mTORC1 pathway downstream molecules, such as S6K1 and 4E-BP1, in in vitro cultured myotubes and malnourished aged rats [43,50-52]. These findings suggest that citrulline regulates muscle performance possibly by activating mTOR in muscle protein synthesis. Finally, the hypothalamus is one of the possible anatomical sites in which citrulline may modulate muscle performance. Hypothalamus is the center of the endocrine and nervous systems. The activity and amount of tyrosine hydroxylase is a rate-limiting enzyme to dopamine synthesis from tyrosine [53- 55]. In aged rats, citrulline supplementation improves the total tyrosine hydroxylase numbers and locomotor activity [56]. Thus, citrulline can regulate daily activities by the dopaminergic pathway. Furthermore, the arginine group (injected L-arginine) increases the total workload of exercise and nNOS-positive cell numbers in the paraventricular nucleus and dorsomedial hypothalamus, decreases the athletic ability of the L-NAME group (injected L-NAME), and exerts no significant effect on the quantity of nNOS-positive cells in rats [57]. These results show that hypothalamic NO participates in regulating the exercise performance of rats. In conclusion, citrulline supplementation obviously enhances animal muscle performance through the NO pathway, mTOR pathway, and the hypothalamus is one of the sites of action.
Citrulline and Intestinal Homeostasis
Citrulline not only adjusts protein synthesis but also exerts certain effects of regulating intestinal homeostasis. The gut is the main source of plasma citrulline; hence, citrulline concentration is an indicator of enterocyte function and quality [58]. Additionally, citrulline can be effectively transported into the intestine through several pathways such as carrier-mediated active transport system in everted sacs of the small intestine [59], the Na+-dependent (system B0,+) and the Na+- independent uptake (systems L and b0,+) in the Caco-2 cells [60], and a neutral amino acid transport system in the hamster intestine [61]. The intestinal homeostasis of animals is responsible for their normal growth and development. The normalization of gut barrier function is one of the important aspects of intestinal homeostasis. The gut barrier is the sum of the structures and functions that prevent intestinal harmful substances, such as bacteria and toxins, from passing through the intestinal mucosa and from being circulated in the blood [62]. The intestinal barrier includes physical, chemical, microbial, and immunological barriers, all of which are related to intestinal health [63]. Citrulline exerts its effects on maintaining intestinal homeostasis by protecting the gut barrier function, promoting intestinal digestion and absorption, and inhibiting intestinal cell apoptosis.
Citrulline and gut barrier function
Citrulline and intestinal physical barrier: Epithelial tight junctions are vital for proper maintenance of intestinal barrier function. Citrulline exerts its modulation effects by regulating the intestinal tight junction and integrity of the intestinal mucosa. Dietary supplementation with L-citrulline enhanced ileal tight junctions in the case of ileum coinfected with malaria and nontyphoidal Salmonella serotypes which impaired intestinal barrier in a mouse model [64]. Additionally, a female C57BL/6J mice model trial which fed with western-style diet and citrulline showed that mice fed a western-style diet + citrulline diet has a significantly higher level of tight junction proteins occludin and zonula occludens-1 in duodenum [65]. Moreover, the trans epithelial electrical resistance ratio is the index that reflects the permeability of cell membranes. Citrulline improves the mean transepithelial electrical resistance ratio of jejunal IPEC-J2 cell monolayers in neonatal piglets and decreases the inulin flux across the hypoxic monolayers after exposure to hypoxia [8]. Hence, citrulline supplementation contributes to the maintenance of the tight junctions and integrity of intestinal epithelial cells. In a murine mucositis model, citrulline administration attenuates damage to the mucosal architecture, decreasing the size of the injured areas and permeability of the small intestine [9]. Additionally, the protective effects of citrulline in the intestinal epithelial cells are prevented in the presence of irreversible NOS inhibitors [8]. Thus, the efficiency of citrulline in intestinal epithelial cells depends on the NO pathway. Therefore, citrulline can regulate the intestinal physical barrier by affecting intestinal mucosa cell junctions, integrity, and permeability.
Citrulline and intestinal immune barrier: The intestinal tract is the largest peripheral immune organ in animals. The intestinal mucosa associated with lymphoid tissue contains more immune cells than other tissues in the body. The intestinal mucosa immune cells secrete various regulatory substances involved in the immune response, including many cytokines (IL-6, IL-10, TNF-a, IFN-γ) and immunoglobulins. IL-10 is an anti-inflammatory cytokine. TNF-a, IFN-γ, and IL-6 possess proinflammatory cytokine effects [10]. Citrulline can affect the intestinal immune barrier through three channels. First, citrulline affects the levels of proinflammatory or anti-inflammatory cytokines. Oral citrulline significantly decreases the jejunal content of TNF-a and IL-6 in the male rat jejunum model of ischemia–reperfusion injury and resistin (an adipose-derived peptide hormone) levels without impairing the secretion of antiinflammatory cytokines (IL-10 and adiponectin) [11,66]; this process provides a safe means of immunomodulation that preserves the anti-inflammatory mediator response. Citrulline supplementation reduces IFN-γ levels and maintains IL-10 levels, which may decrease overall mucosa inflammation [67]. Second, citrulline regulates immunoglobulins, including IgA (including sIgA), IgG, and IgM. Citrulline supplementation increases IgA, IgG, and IgM levels and improves the immunity of suckling piglets [12]. SIgA, a secretory immunoglobulin, participates in the generation of local immune response. It neutralizes the antigen, forms the antigen–antibody complexes, and prevents bacterial adhesion to the intestinal mucosa to prevent the bacteria from entering the intestinal wall [68]. Pretreatment with citrulline can significantly stimulate the intestinal production of sIgA and reduce bacterial translocation [17]. Third, immune cells play critical roles in suppressing the immune response. Neutrophil infiltration is one of the manifestations of inflammation. Citrulline supplementation reduces neutrophil infiltration in the intestinal mucosa [69]. Therefore, neutrophils and macrophages are involved in the regulation process of citrulline in the intestinal mucosa. In summary, citrulline can affect the intestinal immune barrier by regulating cytokine, immunoglobulin, and macrophage levels.
Citrulline and intestinal digestion and absorption
The intestinal tract is the largest digestive organ of the body. Animal life activities depend on intestinal digestion and absorption, which provide nutrition to sustain life. The intestinal epithelium can absorb nutrients and water and block various antigens and toxins [70]. Citrulline supplementation increases the villus length of the duodenum in suckling piglets [12]. Moreover, citrulline promotes the piglet’s digestion and nutrient absorption ability for growth [12]. Citrulline levels can effectively reflect intestinal digestion and absorption capacity [71]. In patients with short bowel syndrome, post-absorptive plasma citrulline concentration is significantly correlated with the net digestive absorption of protein and fat [72]. It also reflects the mass of absorptive enterocyte and the functional absorptive bowel length [72,73]. Therefore, citrulline exerts positive effects on intestinal digestion and absorption, but the exact molecular mechanisms remain unclear.
Citrulline and intestinal cell apoptosis
Intestinal epithelial homeostasis depends on a balance between cell proliferation and apoptosis. Apoptosis, or programmed cell death, is a key factor to control the number of intestinal epithelial cells [74]. Any reason that increases or decreases cell apoptosis may contribute to mucosal atrophy or tumor formation and thus hinder intestinal function [75]. Citrulline has an obvious influence on intestinal cell apoptosis through the NO pathway. NO is synthesized from citrulline, and low NO concentrations protect cells from apoptosis [76]. The antiapoptotic effects of NO are related to the modulation of cyclic nucleotides and ceramide and inhibition of caspases by S-nitrosylation and mitochondrial respiration [13]. In addition, citrulline attenuates jejunal apoptosis through devitalization of the NOS and NF-κB pathways in male Wistar rats [11]. In conclusion, citrulline administration decreases intestinal cell apoptosis.
Citrulline and Antioxidant Function
In normal state, the oxidant and antioxidant abilities of animals are in dynamic equilibrium. Free radicals can make strong reaction with the protein, fat, nucleotides and carbohydrate molecules. When intracellular accumulate lots of free radicals, there will be occurrence of cell apoptosis, cell membrane permeability and barrier function changes and immune damage [77]. Citrulline exhibits certain antioxidant functions. Citrulline can effectively protect plant DNA and enzymes from damage by reactive oxygen species [78]. In animal models, citrulline can effectively reduce the harm caused by oxidative stress. Citrulline supplementation limits lipoprotein oxidation and decreases protein carbonylation and thiobarbituric acid (the final product of lipid peroxidation) reactive substances in elderly rats and in plasma [4,15]. Therefore, citrulline supplementation significantly contributes to antioxidant function.
Conclusion
Citrulline is a highly promising nutrient that has well-defined roles in nitrogen homeostasis and gut homeostasis. In recent years, it has been shown to modulate protein synthesis, intestinal homeostasis and antioxidant capacity (Figure 3). However, the precise mechanism of citrulline in the regulation of intestinal function, e.g., as intestinal barrier, remains unclear. Moreover, the internal molecular mechanism of citrulline for the control of gut digestion and absorption and its antioxidant function are not yet clearly understood. An in-depth study of citrulline to clarify its mechanism of action in maintaining intestinal function will provide new ideas to strengthen animal intestinal health, improve livestock production performance, and promote husbandry development.
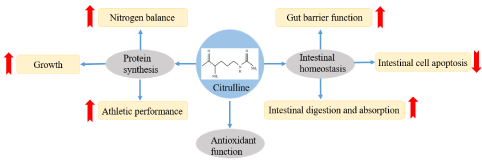
Figure 3: Citrulline: modulation on protein synthesis and intestinal homeostasis.
Acknowledgment
This project was supported by the Program for Discipline Construction in Sichuan Agricultural University (No. 03570126) for financial support.
References
- Rimando AM, Perkins-Veazie PM. Determination of Citrulline in Watermelon Rind. J Chromatogr A. 2005; 1078: 196–200.
- Romero MJ, Platt DH, Caldwell RB, Caldwell RW. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc Drug Rev. 2006; 24: 275– 290.
- Wijnands KAP, Castermans TMR, Hommen MPJ, Meesters DM, Poeze M. Arginine and Citrulline and the Immune Response in Sepsis. Nutrients. 2015; 7: 1426–1463.
- Moinard C, Cynober L. Citrulline: a New Player in the Control of Nitrogen Homeostasis. J Nutr. 2007; 137: 1621S–1625S.
- Bourdon A, Parnet P, Nowak C, Tran NT, Winer N, Darmaun D. L-Citrulline Supplementation Enhances Fetal Growth and Protein Synthesis in Rats with Intrauterine Growth Restriction. J Nutr. 2016; 146: 532–541.
- Tran NT, Amarger V, Bourdon A, Misbert E, Grit I, Winer N, et al. Maternal Citrulline Supplementation Enhances Placental Function and Fetal Growth in a Rat Model of IUGR: Involvement of Insulin-Like Growth Factor 2 and Angiogenic Factors. J Matern Fetal Neonatal Med. 2017; 30: 1906–1911.
- Takeda K, Machida M, Kohara A, Omi N, Takemasa T. Effects of Citrulline Supplementation on Fatigue and Exercise Performance in Mice. J Nutr Sci Vitaminol. 2011; 57: 246–250.
- Chapman JC, Liu Y, Zhu L, Rhoads JM. Arginine and Citrulline Protect Intestinal Cell Monolayer Tight Junctions from Hypoxia-Induced Injury in Piglets. Pediatr Res. 2012; 72: 576–582.
- Antunes MM, Leocádio PCL, Teixeira LG, Leonel AJ, Cara DC, Menezes GB, et al. Pretreatment with L-citrulline Positively Affects the Mucosal Architecture and Permeability of the Small Intestine in a Murine Mucositis Model. JPEN J Parenter Enteral Nutr. 2016; 40: 279–286.
- Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, et al. IL-10, IL-6, and TNF-a: Central Factors in the Altered Cytokine Network of Uremia—the Good, the Bad, and the Ugly. Kidney Int. 2005; 67: 1216–1233.
- Lai CH, Lee CH, Hung CY, Lo HC. Oral Citrulline Mitigates Inflammation and Jejunal Damage via the Inactivation of Neuronal Nitric Oxide Synthase and Nuclear Factor–κB in Intestinal Ischemia and Reperfusion. JPEN J Parenter Enteral Nutr. 2017; 41: 422–435.
- Sun ZJ. Effect of L-citrulline addition level on the growth performance and related physical and biochemical indicators in suckling piglets [dissertation]. Agricultural University of Heibei. 2012.
- Kim PK, Zamora R, Petrosko P, Billiar TR. The Regulatory Role of Nitric Oxide in Apoptosis. Int Immunopharmacol. 2001; 1: 1421–1441.
- Faure C, Morio B, Chafey P, Le Plénier S, Noirez P, Randrianarison Huetz V, et al. Citrulline Enhances Myofibrillar Constituents Expression of Skeletal Muscle and Induces a Switch in Muscle Energy Metabolism in Malnourished Aged Rats. Proteomics. 2013; 13: 2191–2201.
- Bonnefont-Rousselot P, Raynaud-Simon A, Le Plénier S, Cynober L, Moinard C. Citrulline Supplementation is Efficient in Limiting Lipoprotein Oxidation in Healthy Aged Rats. Clin Nutr Suppl. 2010; 5: 105.
- Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Bénazeth S, et al. Almost All About Citrulline in Mammals. Amino acids. 2005; 29: 177–205.
- Breuillard C, Cynober L, Moinard C. Citrulline and Nitrogen Homeostasis: an Overview. Amino acids. 2015; 47: 685–691.
- Wu G, Morris SM. Arginine metabolism: Nitric Oxide and Beyond. Biochem J. 1998; 336: 1–17.
- Bahri S, Zerrouk N, Aussel C, Moinard C, Crenn P, Curis E, et al. Citrulline: from Metabolism to Therapeutic Use. Nutrition. 2013; 29: 479–484.
- Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, et al. Plasma Arginine and Citrulline Kinetics in Adults Given Adequate and Arginine-Free Diets. Proc Natl Acad Sci USA. 1993; 90: 7749–7753.
- Daniel JA, Stockwell-Goering MG, Crane AR, Hernandez CM, Qualley DF, Whitlock BK. Oral Administration of L-citrulline Increases Plasma Concentrations of L-citrulline and Arginine in Horses. J Anim Sci. 2017; 95: 166.
- Wijnands KA, Vink H, Briedé JJ, Van Faassen EE, Lamers WH, Buurman WA, et al. Citrulline a More Suitable Substrate than Arginine to Rrestore NO Production and the Microcirculation during Endotoxemia. PLoS One. 2012; 7: e37439.
- Osowska S, Moinard C, Loi C, Neveux N, Cynober L. Citrulline Increases Arginine Pools and Restores Nitrogen Balance after Massive Intestinal Resection. Gut. 2004; 53: 1781–1786.
- Osowska S, Neveux N, Nakib S, Lasserre V, Cynober L, Moinard C. Impairment of Arginine Metabolism in Rats after Massive Intestinal Resection: Effect of Parenteral Nutrition Supplemented with Citrulline Compared with Arginine. Clin Sci. 2008; 115: 159–166.
- Agarwal U, Didelija IC, Yuan Y, Wang X, Marini JC. Supplemental Citrulline is More Efficient than Arginine in Increasing Systemic Arginine Availability in Mice. J Nutr. 2017; 147: 596–602.
- Marini JC, Didelija IC, Castillo L, Lee B. Plasma Arginine and Ornithine are the Main Citrulline Precursors in Mice Infused with Arginine-Free Diets. J Nutr. 2010; 140: 1432–1437.
- Marini JC, Keller B, Didelija IC, Castillo L, Lee B. Enteral Arginase II Provides Ornithine for Citrulline Synthesis. Am J Physiol Endocrinol Metab. 2011; 300: E188–E194.
- Windmueller HG, Spaeth AE. Source and Fate of Circulating Citrulline. Am J Physiol. 1981; 241: E473–E480.
- Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal Nutrition and Fetal Development. J Nutr. 2004; 134: 2169–2172.
- Ferrell CL, Jenkins TG. Cow Type and the Nutritional Environment: Nutritional Aspects. J Anim Sci. 1985; 61: 725–741.
- Fuquay JW. Heat Stress as it Affects Animal Production. J Anim Sci. 1981; 52: 164–174.
- Noris M, Remuzzi G. Physiology and Pathophysiology of Nitric Oxide in Chronic Renal Disease. Proc Assoc Am Physicians. 1999; 111: 602–610.
- Manson JM, Smith RJ, Wilmore DW. Growth Hormone Stimulates Protein Synthesis During Hypocaloric Parenteral Nutrition. Role of hormonalsubstrate environment. Ann Surg. 1988; 208: 136.
- Sonntag WE, Hylka VW, Meites J. Growth Hormone Restores Protein Synthesis in Skeletal Muscle of Old Male Rats. J Gerontol. 1985; 40: 689– 694.
- Harvey MB, Kaye PL. Insulin Stimulates Protein Synthesis in Compacted Mouse Embryos. Endocrinology. 1988; 122: 1182–1184.
- Kimball SR, Jurasinski CV, Lawrence Jr JC, Jefferson LS. Insulin Stimulates Protein Synthesis in Skeletal Muscle by Enhancing the Association of eIF-4E and eIF-4G. Am J Physiol Cell Physiol. 1997; 272: C754–C759.
- Wu G, Meininger CJ, Knabe DA, Baze FW, Rhoads MJ. Arginine Nutrition in Development, Health and Disease. Curr Opin Clin Nutr Metab Care. 2000; 3: 59–66.
- Reeds PJ, Burrin DG, Davis TA, Fiorotto ML, Stoll B, van Goudoever JB. Protein Nutrition of the Neonate. Proc Nutr Soc. 2000; 59: 87–97.
- Marini JC, Mohammad MA, Didelija IC. Lack of Arginase II Spares Arginine and Restores Growth in Mice. FASEB J. 2017; 31: 652.6.
- Wileman SM, Mann GE, Pearson JD, Baydoun AR. Role of L-citrulline Transport in Nitric Oxide Synthesis in Rat Aortic Smooth Muscle Cells Activated with LPS and Interferon-Gamma. Br J Pharmacol. 2003; 140: 179–185.
- Ventura G, Noirez P, Breuillé D, Godin JP, Pinaud S, Cleroux M, et al. Effect of Citrulline on Muscle Functions During Moderate Dietary Restriction in Healthy Adult Rats. Amino Acids. 2013; 45: 1123–1131.
- Osowska S, Duchemann T, Walrand S, Paillard A, Boirie Y, Cynober L, et al. Citrulline Modulates Muscle Protein Metabolism in Old Malnourished Rats. Am J Physiol Endocrinol Metab. 2006; 291: E582–E586.
- Le Plénier S, Walrand S, Noirt R, Cynober L, Moinard C. Effects of Leucine and Citrulline Versus Non-Essential Amino Acids on Muscle Protein Synthesis in Fasted Rat: a Common Activation Pathway? Amino acids. 2012; 43: 1171–1178.
- Ham DJ, Lynch GS, Koopman R. Citrulline Protects Muscle Cells from Cachectic Stimuli and Preserves Protein Metabolism in Vitro. Clin Nutr. 2013; 32: S117.
- Goron A, Lamarche F, Cunin V, Dubouchaud H, Hourdé C, Noirez P, et al. Synergistic Effects of Citrulline Supplementation and Exercise on Performance in Male Rats: Evidences for Implication of Protein and Energy Metabolisms. Clin Sci. 2017; 131: 775–790.
- Faure C, Raynaud-Simon A, Ferry A, Daugé V, Cynober L, Aussel C, et al. Leucine and Citrulline Modulate Muscle Function in Malnourished Aged Rats. Amino acids. 2012; 42: 1425–1433.
- Stuehr DJ. Mammalian Nitric Oxide Synthases. Biochim Biophys Acta. 1999; 1411: 217–230.
- Ham DJ, Gleeson BG, Chee A, Baum DM, Caldow MK, Lynch GS, et al. L-Citrulline Protects Skeletal Muscle Cells from Cachectic Stimuli through an iNOS-Dependent Mechanism. PLoS One. 2015; 10: e0141572.
- Aguiar AF, Vechetti-Júnior IJ, Souza RW, Piedade WP, Pacagnelli FL, Leopoldo AS, et al. Nitric Oxide Synthase Inhibition Impairs Muscle Regrowth Following Immobilization. Nitric Oxide. 2017; 69: 22–27.
- Le Plenier S, Walrand S, Cynober L, Moinard C. OP049 Direct Action of Citrulline on Muscle Protein Synthesis: Role of the Mtorc1 Pathway. Clin Nutr Supplements. 2011; 6: 20.
- Goron A, Le Plénier S, Archambault E, Cynober L, Moinard C. Citrulline Activates S6K1 and 4E-BP1 in Muscle Cells by both mTOR-Dependent and mTOR-Independent Pathways. Clin Nutr. 2013; 32: S118.
- Le Plénier S, Goron A, Sotiropoulos A, Archambault E, Guihenneuc C, Walrand S, et al. Citrulline Directly Modulates Muscle Protein Synthesis via the PI3K/MAPK/4E-BP1 Pathway in a Malnourished State: Evidence from in Vivo, ex Vivo, and in Vitro Studies. Am J Physiol Endocrinol Metab. 2017; 312: E27–E36.
- Kaufman S. Tyrosine Hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1995; 70: 103–220.
- Cotzias GC, Papavasiliou PS, Fehling C, Kaufman B, Mena I. Similarities Between Neurologic Effects of L-dopa and of Apomorphine. N Engl J Med. 1970; 282: 31–33.
- Axelrod J, Weinshilboum R. Catecholamines. N Engl J Med. 1972; 287: 237–242.
- Moinard C, Tliba L, Diaz J, Le Plénier S, Nay L, Neveux N, et al. Citrulline Stimulates Locomotor Activity in Aged Rats: Implication of the Dopaminergic Pathway. Nutrition. 2017; 38: 9–12.
- Liu H, Xue J. Involvement of Hypothalamic Nitric Oxide Signaling in the Modulation of a Rat’s Exercise Capacity. Neuroreport. 2017; 28: 408–413.
- Peters JHC, Beishuizen A, Keur MB, Dobrowolski L, Wierdsma NJ, van Bodegraven AA. Assessment of Small Bowel Function in Critical Illness: Potential Role of Citrulline Metabolism. J Intensive Care Med. 2011; 26: 105–110.
- Vadgama JV, Evered DF. Characteristics of L-citrulline Transport Across Rat Small Intestine in Vitro. Pediatr Res. 1992; 32: 472–478.
- Bahri S, Curis E, El Wafi FZ, Aussel C, Chaumeil JC, Cynober L, et al. Mechanisms and Kinetics of Citrulline Uptake in a Model of Human Intestinal Epithelial Cells. Clin Nutr. 2008; 27: 672–680.
- Lin EC, Hagihira H, Wilson TH. Specificity of the Transport System for Neutral Amino Acids in the Hamster Intestine. Am J Physiol. 1962; 202: 919–925.
- Swank GM, Deitch EA. Role of the Gut in Multiple Organ Failure: Bacterial Translocation and Permeability Changes. World J Surg. 1996; 20: 411–417.
- Soderholm JD, Perdue MH. II. Stress and Intestinal Barrier Function. Am J Physiol Gastrointest Liver Physiol. 2001; 280: G7–G13.
- Chau JY, Tiffany CM, Nimishakavi S, Lawrence JA, Pakpour N, Mooney JP, et al. Malaria-Associated L-Arginine Deficiency Induces Mast Cell-Associated Disruption to Intestinal Barrier Defenses Against Non-Typhoidal Salmonella bacteremia. Infect Immun. 2013; 81: 3515–3526.
- Sellmann C, Jin CJ, Engstler AJ, De Bandt JP, Bergheim I. Oral Citrulline Supplementation Protects Female Mice from the Development of Non- Alcoholic Fatty Liver Disease (NAFLD). Eur J Nutr. 2017; 56: 2519–2527.
- Asgeirsson T, Zhang S, Nunoo R, Mascarenas C, Dujovny N, Luchtefeld M, et al. Citrulline: a Potential Immunomodulator in Sepsis. Surgery. 2011; 150: 744–751.
- Batista MA, Nicoli JR, dos Santos Martins F, Nogueira Machado JA, Esteves Arantes RM, Pacífico Quirino IE, et al. Pretreatment with Citrulline Improves Gut Barrier after Intestinal Obstruction in Mice. JPEN J Parenter Enteral Nutr. 2012; 36: 69–76.
- Mantis NJ, Rol N, Corthésy B. Secretory IgA’s Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 2011; 4: 603-611.
- Kvidera SK, Horst EA, Lozano EJ, Seibert JT, Al-Qaisi MA, Ross JW, et al. Effects of Supplemental Citrulline on Intestinal Neutrophil Infiltration During Heat Stress and Nutrient Restriction in Growing Pigs. J Anim Sci. 2017; 95: 157.
- Groschwitz KR, Hogan SP. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J Allergy Clin Immunol. 2009; 124: 3–20.
- Papadia C, Sherwood RA, Kalantzis C, Wallis K, Volta U, Fiorini E, et al. Plasma Citrulline Concentration: a Reliable Marker of Small Bowel Absorptive Capacity Independent of Intestinal Inflammation. Am J Gastroenterol. 2007; 102: 1474–1482.
- Crenn P, Coudray–Lucas C, Thuillier F, Cynober L, Messing B. Postabsorptive Plasma Citrulline Concentration is a Marker of Absorptive Enterocyte Mass and Intestinal Failure in Humans. Gastroenterology. 2000; 119: 1496–1505.
- Kabrt J, Hyanek J, Štastná S, Pospíšilová E. Plasma Citrulline Concentration as a Marker of Small Intestine Failure. Biomed Pap. 2003; 146: 75–78.
- Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of Cell Number in the Mammalian Gastrointestinal Tract: the Importance of Apoptosis. J Cell Sci. 1994; 107: 3569–3577.
- Hengartner MO. The Biochemistry of Apoptosis. Nature. 2000; 407: 770–776.
- Mori M. Regulation of Nitric Oxide Synthesis and Apoptosis by Arginase and Arginine Recycling. J Nutr. 2007; 137: 1616S–1620S.
- Lallès JP. Intestinal Alkaline Phosphatase: Multiple Biological Roles in Maintenance of Intestinal Homeostasis and Modulation by Diet. Nutr Rev. 2010; 68: 323–332.
- Akashi K, Miyake C, Yokota A. Citrulline, a Novel Compatible Solute in Drought Tolerant Wild Watermelon Leaves, is an Efficient Hydroxyl Radical Scavenger. FEBS Lett. 2001; 508: 438–442.