1Department of Nutrition and Exercise Physiology, University of Missouri, USA
*Corresponding author: Pam Hinton, Department of Nutrition and Exercise Physiology, University of Missouri, 204 ynn Hall Columbia, MO 65211, USA
Received: October 03, 2014; Accepted: October 20, 2014; Published: October 21, 2014
Citation: Mavrakis Y, Jiang J, Ortinau LC and Hinton PS. Associations between Serum Ferritin and Markers of Glucose Homeostasis and Inflammation in Overweight Young Women. Austin J Nutri Food Sci. 2014;2(9): 1046.
Purpose: Serum ferritin is positively associated with insulin resistance, type 2 diabetes mellitus, and the metabolic syndrome; however, the mechanism underlying this association is not clear, as ferritin is a biomarker of both hepatic iron stores and of inflammation. The objectives of the present study were: 1) to examine the associations between serum ferritin concentrations and markers of inflammation and glucose homeostasis; and 2) to determine the acute changes in serum ferritin following ingestion of a High-Fat, High-Carbohydrate (HFHC) meal challenge in sedentary overweight/obese, but otherwise healthy, women.
Methods: Overweight/obese, but otherwise healthy women (n=16), aged 18-45 years old, participated in this cross-sectional study. Blood was collected prior to and 1, 2, 4, and 6 hour after ingestion of a High-Fat, High-Carbohydrate (HFHC) meal. Inflammatory markers (interlukin-6, IL-6; tumor necrosis factor-α, TNF-α; IL-1β), ferritin, glucose and insulin concentrations were measured in fasting and postprandial blood samples and incremental Area Under Curve (iAUC) was calculated for the 6-hour postprandial period. Paired t-tests were used to determine if postprandial peak concentrations differed from fasting. Relationships between ferritin and markers of inflammation and glucose homeostasis (fasting, peak, iAUC) were evaluated using Pearson's correlation.
Results: Postprandial peak glucose, insulin, IL-6, TNF-α, IL-1β, and ferritin concentrations were significantly greater than fasting; iAUC for IL-6, TNF-α, IL- 1β were positive. Ferritin was not correlated with cytokines, glucose or insulin; IL-6, TNF-α, and IL-1β were significantly correlated.
Conclusion: In overweight, but otherwise healthy women, ferritin appears to be an indicator of iron stores rather than a marker of inflammation. Because ferritin is produced by the liver in response to IL-6,it is possible that we may have observed a greater increase in ferritin had we collected blood at later time points after the HFHC meal.
Keywords: Ferritin; Inflammation; Insulin resistance; Obesity; Iron status
There is considerable evidence from cross-sectional and prospective longitudinal studies that serum ferritin is positively associated with insulin resistance, Type 2 Diabetes Mellitus (T2DM), the metabolic syndrome and its components [1-11]. Although serum ferritin is an indicator of body iron stores, it is also an acute phase protein and is therefore increased during inflammation [12]. Because chronic, low-grade inflammation is thought to play a key role in the pathogenesis of insulin resistance, diabetes and the metabolic syndrome, the biological underpinning of the association between elevated serum ferritin and metabolic dysregulation is unclear. Cross-sectional and meta-analyses suggest that the association might be attributed to both excessive body iron stores and inflammation [1,6,8-10].
Markers of systemic inflammation and oxidative stress increase acutely following ingestion of dietary fat or carbohydrate and the magnitude of the postprandial oxidative and inflammatory stress is proportional to the degree of hypertriglyceridemia and hyperglycemia [13-15]. Postprandial inflammatory and oxidative stress likely have a major impact on public health in the US for several reasons. First, postprandial inflammation appears to play a key role in the pathogenesis of insulin resistance, atherosclerosis and the metabolic syndrome [16]. Second, the majority of Americans spend most of each day in the postprandial state. And, third, because most adults in the US are overweight or obese, they are at risk for an exaggerated postprandial inflammatory response [17]. Although ferritin is an acute phase protein, the ferritin response to ingestion of a High-Fat/ High Carbohydrate (HFHC) meal has not been determined.
The global objective of this project was to explore the reported association between serum ferritin concentrations and serum markers of glucose homeostasis and inflammation in overweight/ obese, sedentary women. The purpose of the study was two-fold:
A cross-sectional study of 16 apparently healthy, overweight/ obese (BMI: ≥25.0 kg/m2) women (18-45 y) was used to test our hypothesis that the biological link between serum ferritin and insulin resistance is systemic inflammation. The study was approved the Institutional Review Board at the University of Missouri.
Potential study participants were recruited from the University of Missouri and Columbia community such as libraries, parks, grocery stores, etc. via mass email, flyers, and newspaper ads. Premenopausal (18-45 y), overweight/obese (BMI ≥25.0 kg/m2), sedentary (< 3 hours purposeful exercise per week) women who were weight stable for the past 3 months (<5-lb. weight change), apparently healthy with no diagnosed cardiovascular disease, hypertension or diabetes, non-smoking, and no dietary supplement use within the past 3 months were eligible to participate in this study. Exclusion criteria included: inflammatory or autoimmune disease; recent surgery, illness or injury; fasting glucose > 6.1 mmol (i.e., 110 mg/dL); use of anti-inflammatory medications; excess alcohol consumption (>3drinks/ day); lactose intolerance; allergy to dairy or egg; irregular sleep/wake cycle; and, pregnant/lactating.
Potential study participants visited the Human Nutrition and Exercise Physiology Laboratory, Department of Nutrition and Exercise Physiology, in McKee Gym to screen for inclusion and exclusion criteria and to review of the informed consent form. Subjects were informed that participation in the study was voluntary and that they might withdraw from participation at any time. If the subjects chose to participate in the study, the informed consent document was signed and witnessed at the second visit. At this visit, participants completed a written medical history and had their glucose measured using a blood glucose meter (Accu-Check); individuals with whose fasting glucose exceeds 6.1mmoL (110 mg/ dL) were excluded. Participants also were provided 7-Day Diet and Physical Activity Log in which to prospectively record their dietary intake and physical activity. Participants recorded physical activity type, intensity, duration, and frequency, and the Compendium of Physical Activities [18] was used to estimate daily energy expended during purposeful exercise. Nutrient intake was assessed using a 7-Day Diet Record (Food Processor 8.0, esha, Salem, OR).
Height and weight were measured using standard procedures for determination of BMI; waist circumference was measured using a tape measure. A whole body dual-energy X-ray absorptiometry scan (DXA, Hologic Delphi W, Shelby Township, MI) was performed to measure body composition after a pregnancy test prior to the DXA scan.
The HFHC meal challenge was performed during the early follicular phase (day 2 - day 5) of the menstrual cycle. Participants reported to the Lab between 06:00 and 08:00 hours after an overnight fast and >24 hour abstention from exercise. Participants had 5 mL of blood drawn from an antecubital vein while in the supine position by a trained phlebotomist for determination of glucose, insulin, ferritin, IL-6, IL-1, and TNF-α.
After the fasted blood draw, participants underwent the HFHC meal challenge for determination of the acute postprandial inflammatory response. The energy and macronutrient content of the HFHC meal was similar to that used in previous studies that elicited postprandial oxidative and inflammatory stress in normal weight or obese individuals [19-23]. The HFHC meal consisted of a milkshake that provided 1.4 kcal/kg of body weight with 51.7 %, 46.4%, and 1.9 % of total energy from carbohydrate, fat and protein, respectively. The shake was prepared from ice cream, milk, and flavored syrup, as described previously by our group with modification to increase the carbohydrate content [24].We used a milkshake rather than the commercially prepared meals used in other investigations for two reasons: 1) to facilitate consumption of the HFHC meal within 15 minutes; and 2) to allow us to more easily and accurately scale the HFHC meal to the body weight of each participant.
Blood (3-ml) was collected 1, 2, 4, and 6 hours following the meal into EDTA tubes. Previous studies have shown that this time frame (5-6 hours post-meal) is appropriate for determination of the postprandial inflammatory response [23,25]. During this time, participants remained sedentary in the Human Nutrition and Exercise Physiology Lab and were allowed to consume only water. Hematocrit was measured in whole blood to control for changes in plasma volume during the 6 hours after the meal challenge. Plasma was separated by centrifugation and stored at -80°C for determination of glucose, insulin, ferritin, IL-6, IL-1, and TNF-α.
Enzyme-Linked Immunoassay (ELISA) was used to measure ferritin (Ramco Labs); CV=7.79%. Milliplex magnetic bead panels were used to measure TNF-α, IL-6, IL-1β, and insulin (EMD Millipore Corporation, Billerica, MA, USA).The primary outcome variables for the postprandial response were incremental Area Under the Curve (iAUC) from pre-meal challenge to 6 hours post and the peak concentrations of cytokines. iAUC was calculated using the trapezoid method [26]; peak concentrations was defined as the highest concentration among the 4 postprandial concentrations (1, 2, 4, 6 hours) after meal intake. Glucose was measured using a colorimetric enzymatic (glucose oxidase) assay (Pointe Scientific, Canton, MI). HOMA-IR (homeostatic model assessment - insulin resistance) was calculated as Glucose (mg/dL) x Insulin (mU/L)/405. A HOMA-IR > 3.80 is considered indicative of insulin resistance. QUICKI (quantitative insulin sensitivity check index) was calculated as 1 / ((log (fasting insulin μU/mL) + log (fasting glucose mg/dL)). A normal QUICKI score is > 0.31.
Descriptive statistics (means ± standard error of mean, frequencies) were performed on demographic and anthropometric variables. Pearson correlations were used to test if there were significant relationships between ferritin and markers of inflammation (fasting, postprandial peak and iAUC) and glucose homeostasis and between fasting ferritin and dietary iron intake; one-tailed tests were used because our hypotheses were directional (i.e., we expected positive correlations between ferritin and cytokines). A repeated measures ANOVA was used to determine if outcomes changed significantly during the 6 hours following the HFHC meal. In addition, paired t-tests were used to determine if peak postprandial concentration differed from pre-HFHC meal fasting concentrations. Statistical significance was set at P<0.05.
Participants were young, obese or overweight, sedentary women (Table 1). No subject was insulin resistant with HOMA-IR and QUICKI values within the normal range (Table 2). All subjects had fasting cytokine concentrations within the normal reference range (Table 2): IL-6, less than 15 pg/mL, TNF-α, less than 5 pg/mL, IL- 1β, less than 5 pg/mL [27]. One subject had undetectable IL-1β, and another subject had both undetectable IL-6 and IL-1β. Undetectable IL-6 and/or IL-1β were assigned a 0 in the statistical analysis.
The mean ferritin concentration of the study participants, which averaged 48 μg/l, was within the normal range (15-150 μg/L) as defined by the World Health Organization [28]. However, ferritin concentrations ranged from 5-167 μg/l in the study. According to the WHO's criteria, four of our subjects likely had depleted iron stores and one subject was at risk for iron overload. Average daily iron intake was 11 mg, which is inadequate compared to the RDA of 18 mg/d for women [28]. In addition, fasting ferritin concentrations were positively correlated with dietary iron intake (r=0.71, p= 0.479).
Fasting ferritin was not significantly correlated with markers of inflammation. However, there were significant correlations between IL-6 and IL-1 β (r=0.536, p=0.032), IL-6 and TNF-α (r=0.538, p=0.032) and between IL-1β and TNF-α (r=0.569, p=0.021). In fasting samples, ferritin and markers of glucose homeostasis were not correlated (data not shown).
A one-way repeated measure ANOVA with time (0 hour, 1 hour, 2 hours, 4 hours and 6 hours) as one factor was performed on hematocrit in all study participants (Table 3). There was no significant time main effect, indicating plasma volume did not change significantly during the HFHCmeal challenge.
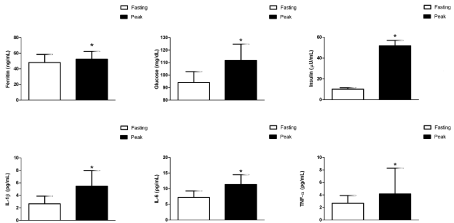
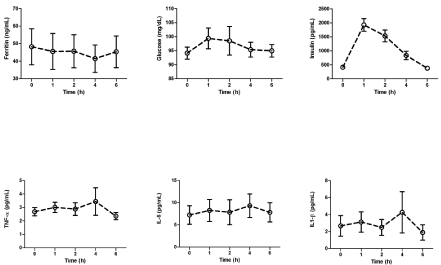
There was a significant time main effect for insulin during the postprandial period, such that insulin concentrations at 1 and 2 hours after ingestion of the HFHC meal were significantly greater than fasting concentrations; postprandial peak glucose and Insulin concentrations were significantly greater than fasting (Figures 1, 2). No significant time main effect was found for the cytokines (Table 2, Figure 1). However, postprandial peak concentrations of TNF-α, IL-1β and IL-6 were all significantly greater than pre-meal concentrations (Figure 2). The percent increase from fasting to peak concentration for the cytokines were as follows: IL-6:47.7 ± 15.6% TNF-α: 40.9 ± 19.1%, and IL-1β: 109.8 ± 70.8%. In addition, the iAUC for the cytokines were positive: IL-6: 6.8 ± 3.5 pg/mL/h; TNF-: 1.8 ±1.7 pg/mL/h; and IL-1 β: 2.6 ± 3.2 pg/mL/h. There was no significant time main effect for ferritin during the postprandial period (Figure 1), and the iAUC was negative (-23.0 ± 10.7 ng/mL/h). Peak ferritin was statistically different than fasting (Figure 2), and the average percent change was 33.9±23.7%.
The peak postprandial concentration of ferritin was not correlated with the peak cytokine or glucose concentrations. Similarly, ferritin iAUC was not correlated with iAUC for the inflammatory markers. There were significant correlations for both peak concentration and iAUC between the cytokines. The postprandial IL-6 peak concentration was significantly correlated with TNF-α (r=0.664, p=0.005) and IL- 1β (r=0.592, p=0.016) postprandial peak concentrations; and, the postprandial TNF-α peak concentration was correlated with IL-1β (r=0.888, p<0.001).The IL-6 iAUC was significantly correlated with TNF-α iAUC (r=0.599, p=0.014) and IL-1β iAUC (r=0.487, p=0.05); and, the TNF-α iAUC was correlated with IL-1β iAUC (r=0.720, p=0.002).
The purpose of the present study was to: 1) examine the associations between serum ferritin concentrations and markers of inflammation (TNF-α, IL-6, IL-1β) and glucose homeostasis (fasting glucose, insulin, QUICKI, HOMA-IR) in sedentary, overweight/obese, but otherwise healthy, women; and, 2) examine the acute changes in serum ferritin following ingestion of a high-fat, high-carbohydrate meal challenge in sedentary overweight/obese, but otherwise healthy, women. We found that ferritin was not correlated with cytokines, glucose, or insulin in either the fasted or state. Although we observed a statistically significant postprandial inflammatory response, as evidenced by positive iAUC for the cytokines, the iAUC for ferritin was negative. Neither peak nor iAUC ferritin was correlated with the cytokines. However, IL-6, TNF-α, and IL-1β were significantly correlated in both fasting and postprandial samples. Although ferritin levels in the subjects were not correlated with inflammatory markers, they were significantly correlated with dietary iron intake. Thus, in these apparently healthy (i.e., not insulin-resistant or diabetic) overweight young women, ferritin does not appear to be a marker of inflammation, but rather an indicator of iron stores.
It is now well established that chronic, low-grade inflammation contributes to obesity-associated insulin resistance and metabolic dysregulation [29] with the adipose tissue being a major source of inflammatory molecules. Despite the recognition that inflammation is important, which inflammatory markers are best to measure is a highly debated question. Of the acute-phase proteins, ferritin is unique in that circulating concentrations reflect hepatic iron stores and the acute-phase response [12]. Because iron is a pro-oxidant and oxidative stress is a mediator of obesity-associated inflammation, it is unclear if the positive associations between ferritin, inflammatory markers, and insulin resistance implicate elevated body iron stores in the causal pathway or simply reflect the chronic inflammatory state. The answer to this question is elusive, as it is difficult to untangle the two possibilities in human observational studies. Moreover, the answer is likely to vary with the subject population. In the present study, fasting ferritin concentrations were not correlated with markers of inflammation or glucose homeostasis, but were correlated with dietary iron intake. The likely explanation for the lack of the significant association is that the participants in the present study had normal glucose control and markers of inflammation. Studies that have reported significant positive associations between ferritin and insulin resistance, T2DM, components of the metabolic syndrome, or markers of inflammation [1-11,30] included participants that varied in metabolic health status so that detection of a significant association was possible. Regardless, sorting out the significance of the positive association between serum ferritin, chronic inflammation, and metabolic dysregulation requires animal studies to control whole body iron status as well as macronutrient overload.
The contribution of repeated postprandial inflammation to the chronic inflammation of obesity, although not trivial, is unknown at this time. Clearly, the postprandial inflammatory response is influenced by participant (e.g., sex, age, body size and composition, fat distribution) and meal characteristics (e.g., size, absolute fat and carbohydrate content, physical form) and by circadian effects [23,31- 35]. Data suggest that high-fat/-sugar induce greater postprandial inflammation than protein and that obesity, in particular visceral adiposity is associated with a greater postprandial inflammatory response than that observed in normal weight, metabolically healthy individuals [23,31-35].
Although the participants in the present study were overweight but otherwise healthy, we observed an inflammatory response during the 6 hours after ingestion of the HFHC meal. Peak concentrations of IL-6, TNF and IL-1b were significantly greater than fasting by ~50-100%. Although peak ferritin was marginally increased relative to fasting, overall ferritin declined during the postprandial period. Because ferritin is produced by the liver in response to other cytokines (e.g., IL-6), it is possible that we may have observed a greater increase in ferritin had we collected blood at later time points after the HFHC meal. To our knowledge, this is the first time the ferritin postprandial response to a meal challenge has been examined. However, others have examined postprandial changes in C-Reactive Protein (CRP), another acute-phase protein produced by the liver, with mixed results. Some studies reported a postprandial increase in CRP in overweight/ obese [36], but not normal weight [37], while others did not [34]. A recent study by Schwander et al. that used a dose-response design to compare the effects of macronutrient content on the postprandial inflammatory response in normal weight and obese men found a significant postprandial increase in IL-6, but not CRP, in the obese men only [38]. Similar to the present study, Schwander et al. looked only at the first 6 hours after the meal challenge, and thus, may have missed an increase in CRP.
Studies of postprandial inflammation require careful design because the outcomes are influenced by many factors related to the participants, meal, and the inflammatory markers selected. Strength of the present study is that the HFHC meal was scaled to body weight as recommended by [35]. The participants all consumed the HFHC meal between 06:00 and 08:00 to reduce circadian variation and were requested to refrain from physical activity for at least 24 hours prior to the meal challenge. Obvious limitations are the metabolically healthy participants, small sample size, and blood sampling only during the first 6 hours of the postprandial period.Despite these limitations, it is still important to note the significant increase in peak glucose, insulin, IL-6, TNF-α, IL-1β, and ferritin concentrations after a HFHC. Our preliminary data in overweight, but otherwise, metabolically healthy, young women suggest that there is a postprandial inflammatory response to a HFHC meal. Additional studies are needed to investigate the association between serum ferritin and the chronic inflammatory state that is thought to play a causal role in the development of many chronic diseases.
Subject descriptive characteristics1.
Age (years) |
26.2 ± 7.1 |
|
Height (cm) |
164.4 ± 5.4 |
|
Race (Caucasian/African-American) |
12/4 |
|
Waist circumference (cm) |
105.2 ± 16.9 |
|
Body weight (kg) |
91.9 ± 23.3 |
|
BMI (kg/m2) |
36.2 ± 13.2 |
|
Lean body mass (kg) |
53.0 ± 73.2 |
|
Fat mass (kg) |
38.7 ± 15.8 |
|
Body fat (%) |
40.9 ± 6.7 |
|
1Data are means ± SE.
Fasting (0 hour) and postprandial concentrations for inflammatory and glucose homeostasis markers at 1 hour, 2 hours, 4 hours, and 6 hours1,2.
Time points |
0 hour |
1 hour |
2 hours |
4 hours |
6 hours |
Inflammatory markers |
|||||
Ferritin (ng/mL) |
48.2 ± 10.3 |
45.6 ± 10.2 |
45.7 ± 9.4 |
41.4 ± 7.9 |
45.4 ± 9.1 |
IL-6 (pg/mL) |
7.2 ± 2.1 |
8.3 ± 2.5 |
7.8 ± 2.8 |
9.3 ± 2.7 |
7.8 ± 2.2 |
TNF-α (pg/mL) |
2.7 ± 0.3 |
3.0 ± 0.4 |
2.9 ± 0.5 |
3.4 ± 1.0 |
2.4 ± 0.3 |
IL-1β (pg/mL) |
2.7 ± 1.2 |
3.1 ± 1.2 |
2.5 ± 1.0 |
4.3 ± 2.4 |
1.9 ± 0.9 |
Glucose homeostasis markers |
|||||
Insulin (μU/mL)3 |
10.2 ± 1.4c |
47.4 ± 5.8a |
37.5 ± 5.4a |
20.3 ± 3.8b |
9.4 ± 0.9c |
Glucose (mg/dL) |
94.1 ± 2.2 |
99.4 ± 3.7 |
98.5 ± 5.1 |
95.4 ± 2.6 |
95.0 ± 2.2 |
HOMA-IR |
2.37±0.30 |
- |
- |
- |
- |
QUICKI |
0.350± 0.008 |
- |
- |
- |
- |
1Data are means ± SE. 1Data were analyzed with one-way repeated measures ANOVA with time (0 hour, 1 hour, 2 hours, 4 hours, 6 hours) as one factor. Post-hoc paired t-tests were performed if there is a significant time main effect. 1Signficant time main effect for insulin (p<0.001). Means with different letter superscripts are significantly different (p<0.05).
Hematocrit of fasting blood samples (0 hour) and blood samples collected at 1 hour, 2 hours, 4 hours and 6 hours after the high-fat, highcarbohydrate meal1,2.
Time points |
% |
0 hour |
40.2 ± 0.7 |
1 hour |
40.1 ± 0.8 |
2 hours |
40.0 ± 0.8 |
4 hours |
40.1 ± 0.8 |
6 hours |
40.3 ± 0.8 |
1Data are means ± SE.2Dataare analyzed with one-way repeated measures ANOVA with time (0 hour, 1 hour, 2 hours, 4 hours, 6 hours) as one factor. No significant time main effect was found.
Fasting (0 hour) and postprandial concentrations of inflammatory and glucose homeostasis markers at 1 hour, 2 hours, 4 hours, and 6 hours1. 1Significant time main effect for insulin (p<0.001); post hoc tests revealed that insulin at 1 and 2 h were significantly greater than 0 h (p<0.05).
Fasting and peak concentrations of glucose, insulin, ferritin, TNF-α, IL-6, and IL-1β.
1Peak concentrations with asterisks are significantly different from fasting concentrations (p<0.05).