Review Article
The Pharmacological Effects of Natural Products and Herbs in Benign Prostatic Hyperplasia
Kao CL1, Hsieh CJ1, Chen CY2 and Liu CM1*
1Department of Nursing, Tzu Hui Institute of Technology, Taiwan
2School of Medical and Health Sciences, Fooyin University, Taiwan
*Corresponding author: Liu CM, Department of Nursing, Tzu Hui Institute of Technology, Pingtung County 92641, Taiwan
Received:July 04, 2014; Accepted: November 26, 2014; Published: November 29, 2014
Citation: Kao CL, Hsieh CJ, Chen CY and Liu CM. The Pharmacological Effects of Natural Products and Herbs in Benign Prostatic Hyperplasia. Austin J Nutri Food Sci. 2014;2(10): 1054. ISSN: 2381-8980.
Abstract
Benign Prostatic Hyperplasia (BPH) is a common disease in older men. The symptoms of BPH is urination frequency, frequent night urination and a sense of urgency. Herbal medicine is commonly used as a treatment for BPH. Herbs and natural products are acceptable for improving quality of life or adjuvant use in Western countries. In this review article, we will introduce the pharmacological action of herbs and natural products in controlling the lower urinary tract symptoms associated with BPH.
Method: This review gathers the information from electronic and scientific literature database such as Pubmed, Medline, ScienceDirect and so on. In this review, we focus on the pharmacological effects of Serenoa repenes, lycopene (extraction from tomato), Urtica dioica (root), Pygeum africanum (bark).
Conclusions: Phytotherapy is well acceptable for the alleviating the symptoms of BPH. There are a lot of plants with multi-pharmacological effects in the controlling BPH. The active ingredients could be developed as a new drug in the future.
Keyword: Benign prostatic hyperplasia; Serenoa repens; Urtica dioica; Lycopene; Pygeum africanum; Apoptosis
Introduction
The development of BPH (benign prostatic hyperplasia) and LUTS (Lower urinary tract symptoms) correlates with age [1]. The symptoms of BPH include increased urination frequency, frequent night urination and a sense of urgency. The phenomenon is mainly due to enlarged prostate bladder neck oppressed. It is well known that the treatment options include α1-adrenoreceptor antagonists, 5α-reductase inhibitors, transurethral resection of the prostate, transurethral microwave thermotherapy and herbal treatments. α1- adrenoreceptor antagonists are currently the preferred first-line therapy for all men with moderate or severe LUTS/BPH [2]. α1- adrenoreceptor antagonists relax prostatic and bladder neck smooth muscle and partially relieve LUTS by improving bladder outlet obstruction.
5α-reductases are a family of enzymes and transform steroid precursors into active hormones and neurosteroids. The 5α-reductases catalyzed reaction is the rate limiting step in the conversion of testosterone to dihydrotestosterone (DHT) [3]. Testosterone and DHT induces stromal and epithelial cells growth. 5α-reductase inhibitor such as finasteride inhibits 5α-reductase blocking the conversion of testosterone to DHT. 5α-reductase inhibitors, α1- adrenoceptor antagonists and plant extracts are available to relieve symptoms of BPH. In the USA, phototherapies are available for BPH as dietary supplements. In this review article, we will focus on plant extracts and reveal the pharmacological mechanisms of actions.
Serenoa repens
Serenoa repenes is known as saw palmetto and classified as in the genus Serena. The fruits Saw Palmetto Extraction (SPE) is used for the symptomatic treatment of BPH. SPE contain fatty acids, glycerides, sterols and sitosterol derivates. Serenoa repens (Permixon®) is a mixture of various compounds from an n-hexane lipido/sterolic extract of American dwarf palm tree (saw palmetto, Serenoa repens) [4].
The lipido/sterolic extract of Serenoa repens has anti-inflammatory activity, anti-androgen properties and anti-edema effects in the prostate [4-9]. Oleic and lauric acids of SPE have both 5α-reductase (1 and 2) inhibition activities and play an important role in the treatment of BPH [10]. Oleic and lauric acids of SPE also has α1-adrenergic, muscarinic and 1,4-dihydropyridine receptors binding activity in rat tissues [11]. Fatty acid of SPE causes inhibition of prostatic smooth muscle contractions [12]. A clinical study has reported a significant decrease testosterone in BPH patients receiving Permixon (320 mg/ day) for 3 months [13]. It is suggested that Permixon has 5α-reductase (1 and 2) inhibition in human prostate.
Proliferation and apoptosis are physiological mechanisms involved in the maintenance of prostate function. The imbalance of apoptosis and proliferation can cause BPH. SPE has anti-proliferative activity and apoptotic activity in primary prostate cells and BPH patients [14-16]. Moreover, SPE increases bax/bcl-2 ratio and caspase-3 activity in prostatic specimens from BPH patients [14].
Recent studies have shown chronic prostatic inflammation play a major role in the development of BPH [17,18]. Permixon potently antagonizes the cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) metabolites production or suppress the expression of inflammatory mediators such as MCP-1 and VCAM-1 [19,20]. The metabolites form 5-lipoxygenase was inhibited at a concentration of 5 μg/mL.
The interaction between herb and drug has been concerned because herbs have a lot of constituents. Until now, no drug interaction with SPE has been published. Studies have shown that SPE had no significant effect on cytochrome P-450 (CYP) such as CYP1A2, CYP2D6, CYP2E1, or CYP3A4 [21,22].
Serenoa repens in combination with selenium and lycopene is more effective than saw palmetto alone in controlling BPH [18,23- 25]. Study has shown that serenoa repens /selenium/lycopene decrease prostatic growth and the effect might contribute from induction of programmed cell death. Serenoa repens /selenium/lycopene has potent anti-inflammatory. Serenoa repens /selenium/lycopene caused a greater inhibitory effect on the expression of COX-2, 5-LOX and iNOS than palmetto alone in vitro study [18].
Lycopene
Lycopene is present in the red vegetables and fruits, which is the red carotenoid pigment. Tomatoes and its products are one of the main sources for lycopene. Oxidative stress damages to biomolecules such as DNA, lipids and proteins, leading to several chronic diseases, including inflammatory disease, cardiovascular disease and cancer [26,27]. Chronic and acute inflammation can promote the proliferation of prostate epithelial cells through oxidative stress [28]. By assessing malondialdehyde (MDA), the marker of lipid peroxidation, results showed the evidence of association of oxidative stress in BPH patients [29,30].
The consumption of tomatoes and tomato products significantly reduced plasma prostate specific antigen (PSA) levels in patients with benign prostate hyperplasia [31]. A controlled clinical study reported that lycopene supplementation at a dose of 15 mg/d for 6 month may increase plasma lycopene concentration and inhibit the serum levels of PSA in BPH patients [32].
Lycopene has the ability to reduce oxidative DNA damage [33]. Lycopene is able to against the reactive oxygen species, including hydrogen peroxide, nitrogen dioxide, thiyl (RS) and sulphonyl (RSO2) radicals [34-37]. Evidence suggests that lycopene increased the levels of non-enzmymatic antioxidant such as vitamin C, vitamin E and reduced glutathione, and enhancing the activity of the phase II detoxifying enzymes Glutathione Peroxidase (GPx), glutathione-S-transferase (GST) and Glutathione Reductase (GR) [38-40]. Insulin-like growth factor 1 (IGF-I) overexpression causes prostatic epithelial neoplasia [41] and facilitate the emergence of hyperplastic lesions in transgenic mice [38,42].
Lycopene supplementation reduced expression of IGF-I and inflammatory markers such as IL-1β, L-selectin and MIP-2 (macrophage inflammatory proteins) in normal rats prostate tissue [43]. Experimental studies have shown that lycopene interfere with the cell growth in human prostate, mammary and lung cancer cells [44,45]. Lycopene inhibits cell growth cells via regulation cell cycle-related genes, such as cyclins D1 and E, Cyclin-Dependent Kinases (CDK) 2 and CDK4 and p27 [46-48]. Similar results were obtained in inhibition of the growth of normal prostate epithelial cells via down-regulation of cyclin D1 protein expression and consequent cell cycle arrest at the G0/G1 phase [49]. Enhanced expression of anti-apoptotic proteins like bcl-2, surviving, leading to a growth imbalance in cell proliferation might promote prostatic hyperplasia [50,51]. Tomato consumption increased the apoptotic index in hyperplastic and neoplastic cells of prostate cancer patients [52], but it has not been found in benign prostate hyperplasia cells [53].
Urtica dioica
Urtica dioica is also called common nettle or stinging nettle native to Europe, Asia, northern Africa, and North America. The plant is used for folk medicine against various diseases. The roots of Urtica dioica (Urticaceae) extraction is currently for the treatment of BPH [54-57]. The roots of Urtica dioica extraction contain sterols, glycosides, glycoproteins, polysaccharides, fatty acids, and so on. Studies have shown that extractions have anti-proliferation activity associated with sex hormone binding globulin, epidermal growth factor, prostate steroid membrane receptors binding activity and weak 5α-reductase inhibition [58,59]. An in vitro study has shown that the extraction decreased serum testosterone and PSA levels against prostatic hyperplasia induced by testosterone [60]. There is no drug interaction with the extraction to date. The benefit of extraction in the treatment of BPH should be conducted more clinical studies in the future.
Pygeum africanum
Pygeum africanum is extracted from the bark of the African plum tree. It has been used in Europe since 1969 as a treatment of symptomatic BPH [61]. Preliminary clinical studies investigated that Pygeum africanum may moderate urologic symptoms and flow measures [62]. In animal models, Pygeum africanum extract may significantly improve disorders of micturition, effectively inhibit enlargement of the prostate and reduce prostatic weight [63,64]. A study showed that Pygeum africanum extract has 5α-reductase inhibition activity [65]. In addition, Pygeum africanum extract antagonizes 5-lipoxygenase metabolite production in BPH [66]. Moreover, some evidence pointed that Pygeum africanum extract has anti-proliferation [67-69] and apoptotic activity in prostatic myofibroblasts and fibroblasts [69].
Conclusions
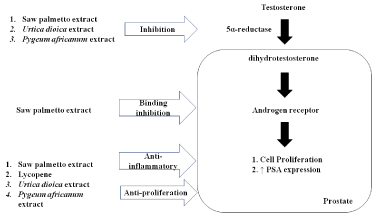
The pharmacological effects of plant extractions are summarized in Table 1 and Figure 1. There are more and more plant extracts and natural products in the treatment of BPH. Herbs and natural products are usually combined with prescription drugs. Therefore, the active ingredients of natural products should be analyzed and the drug interaction should be concerned. Fortunately, phytotherapy is well tolerated and no serve drug interaction reported by users so far. More clinical studies should be conducted in the future.
References
- Trueman P, Hood SC, Nayak US, Mrazek MF. Prevalence of lower urinary tract symptoms and self-reported diagnosed 'benign prostatic hyperplasia', and their effect on quality of life in a community-based survey of men in the UK. BJU international. 1999;83: 410-415.
- Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013; 64: 118-140.
- Traish AM. 5α-reductases in human physiology: an unfolding story. Endocr Pract. 2012; 18: 965-975.
- Carilla E, Briley M, Fauran F, Sultan C, Duvilliers C. Binding of Permixon, a new treatment for prostatic benign hyperplasia, to the cytosolic androgen receptor in the rat prostate. J Steroid Biochem. 1984; 20: 521-523.
- Sultan C, Terraza A, Devillier C, Carilla E, Briley M, Loire C, et al. Inhibition of androgen metabolism and binding by a liposterolic extract of "Serenoa repens B" in human foreskin fibroblasts. J Steroid Biochem. 1984; 20: 515-519.
- el-Sheikh MM, Dakkak MR, Saddique A. The effect of Permixon on androgen receptors. Acta Obstet Gynecol Scand. 1988; 67: 397-399.
- Di Silverio F, D'Eramo G, Lubrano C, Flammia GP, Sciarra A, Palma E, et al. Evidence that Serenoa repens extract displays an antiestrogenic activity in prostatic tissue of benign prostatic hypertrophy patients. Eur Urol. 1992; 21: 309-314.
- Tarayre JP, Delhon A, Lauressergues H, Stenger A, Barbara M, Bru M, et al. [Anti-edematous action of a hexane extract of the stone fruit of Serenoa repens Bartr]. Ann Pharm Fr. 1983; 41: 559-570.
- Paubert-Braquet M, Mencia Huerta JM, Cousse H, Braquet P. Effect of the lipidic lipidosterolic extract of Serenoa repens (Permixon) on the ionophore A23187-stimulated production of leukotriene B4 (LTB4) from human polymorphonuclear neutrophils. Prostaglandins Leukot Essent Fatty Acids. 1997; 57: 299-304.
- Rösler TW, Matusch R, Weber B, Schwarze B. Analysis of the hydrodistillate from the fruits of Serenoa repens. Planta Med. 2009; 75: 184-186.
- Abe M, Ito Y, Suzuki A, Onoue S, Noguchi H, Yamada S. Isolation and pharmacological characterization of fatty acids from saw palmetto extract. Anal Sci. 2009; 25: 553-557.
- Chua T, Eise NT, Simpson JS, Ventura S. Pharmacological characterization and chemical fractionation of a liposterolic extract of saw palmetto (Serenoa repens): effects on rat prostate contractility. J Ethnopharmacol. 2014; 152: 283-291.
- Di Silverio F, Monti S, Sciarra A, Varasano PA, Martini C, Lanzara S, et al. Effects of long-term treatment with Serenoa repens (Permixon) on the concentrations and regional distribution of androgens and epidermal growth factor in benign prostatic hyperplasia. Prostate 1998; 37: 77-83.
- Vela-Navarrete R, Escribano-Burgos M, Farre AL, Garcia-Cardoso J, Manzarbeitia F, Carrasco C. Serenoa repens treatment modifies bax/bcl-2 index expression and caspase-3 activity in prostatic tissue from patients with benign prostatic hyperplasia. J Urol. 2005; 173: 507-510.
- Vacherot F, Azzouz M, Gil-Diez-De-Medina S, Colombel M, De La Taille A, Lefrere Belda MA, et al. Induction of apoptosis and inhibition of cell proliferation by the lipido-sterolic extract of Serenoa repens (LSESr, Permixon in benign prostatic hyperplasia. Prostate. 2000; 45: 259-266.
- Bayne CW, Ross M, Donnelly F, Habib FK. The selectivity and specificity of the actions of the lipido-sterolic extract of Serenoa repens (Permixon) on the prostate. J Urol. 2000; 164: 876-881.
- Silvestri I, Cattarino S, Aglianò A, Nicolazzo C, Scarpa S, Salciccia S, et al. Effect of Serenoa repens (Permixon®) on the expression of inflammation-related genes: analysis in primary cell cultures of human prostate carcinoma. J Inflamm (Lond). 2013; 10: 11.
- Bonvissuto G, Minutoli L, Morgia G, Bitto A, Polito F, Irrera N, et al. Effect of Serenoa repens, lycopene, and selenium on proinflammatory phenotype activation: an in vitro and in vivo comparison study. Urology. 2011; 77: 248.
- Latil A, Libon C, Templier M, Junquero D, Lantoine-Adam F, Nguyen T. Hexanic lipidosterolic extract of Serenoa repens inhibits the expression of two key inflammatory mediators, MCP-1/CCL2 and VCAM-, in vitro. BJU Int. 2012; 110: E301-307.
- Iglesias-Gato D, Carsten T, Vesterlund M, Pousette A, Schoop R, Norstedt G. Androgen-independent effects of Serenoa repens extract (Prostasan®) on prostatic epithelial cell proliferation and inflammation. Phytother Res. 2012; 26: 259-264.
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Carrier J, et al. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clinical pharmacology and therapeutics. 2004; 76: 428-440.
- Markowitz JS, Donovan JL, Devane CL, Taylor RM, Ruan Y, Wang JS, et al. Multiple doses of saw palmetto (Serenoa repens) did not alter cytochrome P450 2D6 and 3A4 activity in normal volunteers. Clin Pharmacol Ther. 2003; 74: 536-542.
- Minutoli L, Altavilla D, Marini H, Rinaldi M, Irrera N, Pizzino G, et al. Inhibitors of apoptosis proteins in experimental benign prostatic hyperplasia: effects of serenoa repens, selenium and lycopene. J Biomed Sci. 2014; 21: 19.
- Morgia G, Cimino S, Favilla V, Russo GI, Squadrito F, Mucciardi G, et al. Effects of Serenoa repens, selenium and lycopene (Profluss) on chronic inflammation associated with benign prostatic hyperplasia: results of "FLOG" (Flogosis and Profluss in Prostatic and Genital Disease), a multicentre Italian study. Int Braz J Urol. 2013; 39: 214-221.
- Altavilla D, Bitto A, Polito F, Irrera N, Marini H, Arena S, et al. The combination of Serenoa repens, selenium and lycopene is more effective than serenoa repens alone to prevent hormone dependent prostatic growth. J Urol. 2011; 186: 1524-1529.
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006; 160: 1-40.
- Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. CMAJ. 2000; 163: 739-744.
- Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev Urol. 2011; 13: 147-150.
- Aryal M, Pandeya A, Gautam N, Baral N, Lamsal M, Majhi S, et al. Oxidative stress in benign prostate hyperplasia. Nepal Med Coll J. 2007; 9: 222-224.
- Aryal M, Pandeya A, Bas BK, Lamsal M, Majhi S, Pandit R, et al. Oxidative stress in patients with benign prostate hyperplasia. JNMA J Nepal Med Assoc. 2007; 46: 103-106.
- Edinger MS, Koff WJ. Effect of the consumption of tomato paste on plasma prostate-specific antigen levels in patients with benign prostate hyperplasia. Braz J Med Biol Res. 2006; 39: 1115-1119.
- Schwarz S, Obermüller-Jevic UC, Hellmis E, Koch W, Jacobi G, Biesalski HK. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J Nutr. 2008; 138: 49-53.
- Park YO, Hwang ES, Moon TW. The effect of lycopene on cell growth and oxidative DNA damage of Hep3B human hepatoma cells. Biofactors. 2005; 23: 129-139.
- Böhm F, Edge R, Burke M, Truscott TG. Dietary uptake of lycopene protects human cells from singlet oxygen and nitrogen dioxide - ROS components from cigarette smoke. J Photochem Photobiol B. 2001; 64: 176-178.
- Tang X, Yang X, Peng Y, Lin J. Protective effects of lycopene against H2O2-induced oxidative injury and apoptosis in human endothelial cells. Cardiovasc Drugs Ther. 2009; 23: 439-448.
- Mortensen A, Skibsted LH. Relative stability of carotenoid radical cations and homologue tocopheroxyl radicals. A real time kinetic study of antioxidant hierarchy. FEBS Lett. 1997; 417: 261-266.
- Mortensen A, Skibsted LH, Sampson J, Rice-Evans C, Everett SA. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997; 418: 91-97.
- Wertz K, Siler U, Goralczyk R. Lycopene: modes of action to promote prostate health. Arch Biochem Biophys. 2004; 430: 127-134.
- Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, et al. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005; 4: 177-186.
- Velmurugan B, Bhuvaneswari V, Nagini S. Antiperoxidative effects of lycopene during N-methyl-N'-nitro-N-nitrosoguanidine-induced gastric carcinogenesis. Fitoterapia. 2002; 73: 604-611.
- DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltrán L, et al. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci U S A. 2000; 97: 3455-3460.
- Kaplan-Lefko PJ, Sutherland BW, Evangelou AI, Hadsell DL, Barrios RJ, Foster BA, et al. Enforced epithelial expression of IGF-1 causes hyperplastic prostate growth while negative selection is requisite for spontaneous metastogenesis. Oncogene 2008; 27: 2868-2876.
- Herzog A, Siler U, Spitzer V, Seifert N, Denelavas A, Hunziker PB, et al. Lycopene reduced gene expression of steroid targets and inflammatory markers in normal rat prostate. FASEB J. 2005; 19: 272-274.
- Pastori M, Pfander H, Boscoboinik D, Azzi A. Lycopene in association with alpha-tocopherol inhibits at physiological concentrations proliferation of prostate carcinoma cells. Biochem Biophys Res Commun. 1998; 250: 582-585.
- Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, et al. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer. 1995; 24: 257-266.
- Nahum A, Hirsch K, Danilenko M, Watts CK, Prall OW, Levy J, et al. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27 (Kip1) in the cyclin E-cdk2 complexes. Oncogene. 2001; 20: 3428-3436.
- Karas M, Amir H, Fishman D, Danilenko M, Segal S, Nahum A, et al. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr Cancer. 2000; 36: 101-111.
- Hwang ES, Bowen PE. Cell cycle arrest and induction of apoptosis by lycopene in LNCaP human prostate cancer cells. J Med Food. 2004; 7: 284-289.
- Obermüller-Jevic UC, Olano-Martin E, Corbacho AM, Eiserich JP, van der Vliet A, Valacchi G, et al. Lycopene inhibits the growth of normal human prostate epithelial cells in vitro. J Nutr. 2003; 133: 3356-3360.
- Kyprianou N, Tu H, Jacobs SC. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol. 1996; 27: 668-675.
- Shariat SF, Ashfaq R, Roehrborn CG, Slawin KM, Lotan Y. Expression of survivin and apoptotic biomarkers in benign prostatic hyperplasia. J Urol. 2005; 174: 2046-2050.
- Kucuk O, Sarkar FH, Djuric Z, Sakr W, Pollak MN, Khachik F, et al. Effects of lycopene supplementation in patients with localized prostate cancer. Exp Biol Med (Maywood). 2002; 227: 881-885.
- Soares Nda C, Teodoro AJ, Oliveira FL, Santos CA, Takiya CM, Junior OS, et al. Influence of lycopene on cell viability, cell cycle, and apoptosis of human prostate cancer and benign hyperplastic cells. Nutr Cancer. 2013; 65: 1076-1085.
- Pavone C, Abbadessa D, Tarantino ML, Oxenius I, Lagana A, Lupo A, et al. [Associating Serenoa repens, Urtica dioica and Pinus pinaster. Safety and efficacy in the treatment of lower urinary tract symptoms. Prospective study on 320 patients]. Urologia. 2010; 77: 43-51.
- Lopatkin N, Sivkov A, Schlafke S, Funk P, Medvedev A, Engelmann U. Efficacy and safety of a combination of Sabal and Urtica extract in lower urinary tract symptoms--long-term follow-up of a placebo-controlled, double-blind, multicenter trial. Int Urol Nephrol. 2007; 39: 1137-1146.
- Sökeland J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome. BJU Int. 2000; 86: 439-442.
- Safarinejad MR. Urtica dioica for treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005; 5: 1-11.
- Hryb DJ, Khan MS, Romas NA, Rosner W. The effect of extracts of the roots of the stinging nettle (Urtica dioica) on the interaction of SHBG with its receptor on human prostatic membranes. Planta Med. 1995; 61: 31-32.
- Wagner H, Geiger WN, Boos G, Samtleben R. Studies on the binding of Urtica dioica agglutinin (UDA) and other lectins in an in vitro epidermal growth factor receptor test. Phytomedicine. 1995; 1: 287-290.
- Nahata A, Dixit VK. Ameliorative effects of stinging nettle (Urtica dioica) on testosterone-induced prostatic hyperplasia in rats. Andrologia. 2012; 44 Suppl 1: 396-409.
- Wilt T, Ishani A, Mac Donald R, Rutks I, Stark G. Pygeum africanum for benign prostatic hyperplasia. The Cochrane database of systematic reviews 2002: CD001044.
- Ishani A, MacDonald R, Nelson D, Rutks I, Wilt TJ. Pygeum africanum for the treatment of patients with benign prostatic hyperplasia: a systematic review and quantitative meta-analysis. Am J Med. 2000; 109: 654-664.
- Choo MS, Bellamy F, Constantinou CE. Functional evaluation of Tadenan on micturition and experimental prostate growth induced with exogenous dihydrotestosterone. Urology. 2000; 55: 292-298.
- Yoshimura Y, Yamaguchi O, Bellamy F, Constantinou CE. Effect of Pygeum africanum tadenan on micturition and prostate growth of the rat secondary to coadministered treatment and post-treatment with dihydrotestosterone. Urology. 2003; 61: 474-478.
- Hartmann RW, Mark M, Soldati F. Inhibition of 5α-reductase and aromatase by PHL-00801 (Prostatonin®), a combination of PY102 (Pygeum africanum) and UR102 (Urtica dioica) extracts. Phytomedicine. 1996; 3: 121-128.
- Paubert-Braquet M, Cave A, Hocquemiller R, Delacroix D, Dupont C, Hedef N, et al. Effect of Pygeum africanum extract on A23187-stimulated production of lipoxygenase metabolites from human polymorphonuclear cells. J Lipid Mediat Cell Signal. 1994; 9: 285-290.
- Yablonsky F, Nicolas V, Riffaud JP, Bellamy F. Antiproliferative effect of Pygeum africanum extract on rat prostatic fibroblasts. J Urol. 1997; 157: 2381-2387.
- Boulbès D, Soustelle L, Costa P, Haddoum M, Bali JP, Hollande F, et al. Pygeum africanum extract inhibits proliferation of human cultured prostatic fibroblasts and myofibroblasts. BJU Int. 2006; 98: 1106-1113.
- Quiles MT, Arbos MA, Fraga A, de Torres IM, Reventos J, Morote J. Antiproliferative and apoptotic effects of the herbal agent Pygeum africanum on cultured prostate stromal cells from patients with benign prostatic hyperplasia (BPH). Prostate. 2010; 70: 1044-1053.
Table 1
The pharmacological effects of plant extracts in the treatment of BPH.
Name |
Main mechanisms of action |
Saw Palmetto extraction |
5α-reductase inhibition; blockage androgen receptors; anti-proliferation; anti-inflammatory activity; prostate smooth muscle relaxation; |
Lycopene |
5α -reductase inhibition; anti-proliferation; anti-inflammatory; anti-oxidant activity |
Urtica dioica extract |
Decreased action of sex hormones; weak 5α -reductase inhibition |
Pygeum africanum extract |
5α -reductase inhibation; anti-inflammatory activity |
Mechanisms of pharmacological action of plant extract.