1Department of Tea Science, Zhejiang University, China
2CSIRO Animal, Food and Health Sciences, Australia
*Corresponding author: Jian-Hui Ye, Zhejiang University Tea Research Institute, Hangzhou 310058, China
Received: November 05, 2014; Accepted: November 17, 2014; Published: November 18, 2014
Citation: Ye JH, Wijesundera C and Shi M. Effects of Agronomic and Oil Processing Conditions on Natural Antioxidative Phenolics in Olive (Oleaeuropaea L.). Austin J Nutri Food Sci. 2014;2(9): 1050. ISSN: 2381-8980.
Olive fruits contain appreciable amounts of phenolic compounds which are valuable antioxidants and bioactives. Secoiridoids, such as oleuropein, demethyloleuropein, oleuropein aglycone and ligstroside are the predominant classes of phenolic compounds in intact olive fruits, while 4-Hydroxyphenylethanol (4-HPEA), 3,4-Dihydroxyphenylethanol (3,4-DHPEA), the dialdehydic form of decarboxymethyl elenolic acid linked to 3,4-DHPEA (3,4-DHPEA-EDA) and oleuropein aglycone (3,4-DHPEA-EA) are the principal phenolic constituents of Virgin Olive Oil (VOO). The phenolic compounds originally present in the olive fruit are substantially changed by the conditions of oil processing (crushing, malaxation and phase separation) and distributed among the final product (VOO) and by-products in various ways. They may undergo further changes during storage. This paper reviews the effects of the processing steps on the transfer of phenolic compounds from intact fruit to the final product (VOO), as well as the effects of agronomic factors (cultivar, maturity and irrigation) on the phenolic constituents of olive fruits at harvest.
Keywords: Olives; Olive oil; Phenols; Milling; Extraction/separation
The olive tree (Oleaeuropaea L.) is a small tree that grows natively in the tropical and subtropical regions of the world, such as the eastern Mediterranean Basin, the coastal areas of south Eastern Europe, northern Africa and Australia. As a member of the family Oleaceae, the olive tree is best known for its fruit which is the primary source of olive oil. The olive tree has a long history of nutritional and medicinal uses in the Mediterranean region, and olive leaves and olive-leaf extracts in particular have been claimed to have antioxidant, antimicrobial and cardio protective properties which have been attributed to their phenolic constituents [1,2].
It has been widely acknowledged that consumption of olive oil exerts benefits on human health, including reducing the risk coronary heart disease, preventing several types of cancers, as well as enhancing immune and inflammatory responses [3-5]. This is evidenced by a reduced incidence of cardiovascular diseases and certain cancers (e.g. breast cancer and colorectal cancer) in the traditional "Mediterranean diet" countries in which olive oil is the major source of dietary fat, in comparison with other regions [4,6]. The nutritional and medicinal properties of olive oil have been attributed to the high content of monounsaturated fatty acids and the presence of functional bioactives such as oleic acid, tocopherols, carotenoids, phospholipids and phenolic compounds [7,8]. Recently, it has become increasingly evident that Virgin Olive Oil (VOO), which is the main source of fat in the Mediterranean diet, is more than a monounsaturated-rich source, and its phenolics could account for much of its nutrigenomic protective effects of the Mediterranean diet [3,5,9]. Numerous studies testify the physiological functions of olive phenolic isolates in vivo and in vitro [10-12]. In addition to the beneficial role in human health, the olive phenolics impart unique sensory characteristics to the oils and enhance oil stability [13].
The total concentration of phenolic compounds in Virgin Olive Oil (VOO) generally ranges from 40 to 1000 mg/kg [14], with various concentrations of individual phenolic compounds being present. These phenolic compounds in VOO originate from those originally present in the olive fruits used for oil extraction but are substantially modified depending on the conditions used for oil extraction and subsequent storage [15]. The phenolic composition of olive fruit at harvest is influenced by agronomic factors [16]. Only a small fraction (approximately 2%) of the phenolic compounds present in the olive fruit is transferred to the extracted oil, the rest (98%) is either retained in the pulp or washed off with the wastewater [17,18]. Yet, the phenolic composition of the olive fruit at harvest is probably the most important variable involved in the phenolic composition of the final VOO [19].
As the type and concentration of phenolic compounds present in VOO are key determinants of both the bioactivity and sensory characteristics of the oil, it is important to understand how these compounds are impacted by agronomic conditions used to grow the fruit as well as the processing conditions used for oil extraction. Whilst, several papers have been published reviewing the effects of some of these variables often on the total phenolic concentration of VOO [19], few papers are available on the effect of each variable on individual phenolic components. Additionally, the transformations involving individual phenolics during oil extraction and their transfer routes to the final product (VOO) are still unclear. This review aims to fulfil this gap by discussing the transfer of phenolics from olive fruit to VOO by dividing the transfer path into two segments: preharvest period of olive fruit and postharvest oil processing. In this approach, to the extent possible, the effects of the agronomic factors (olive cultivar, maturity and irrigation) and oil processing variables (crushing, malaxation, phase separation and storage conditions) on individual phenolic components are reviewed.
Olive fruit generally contains 50% water, 1.6% protein, 22% oil, 19.1% carbohydrate, 5.8% cellulose, 1.5% inorganic substances and 1-3% phenolic compounds [20]. Phenolic compounds in olive fruits are primarily classified into phenolic acids, phenolic alchohols, flavonoids and the glucosides, secoiridoids and the hydrolytic derivatives, anthocyanins as well as hydroxycinnamic acid derivatives (Table 1).
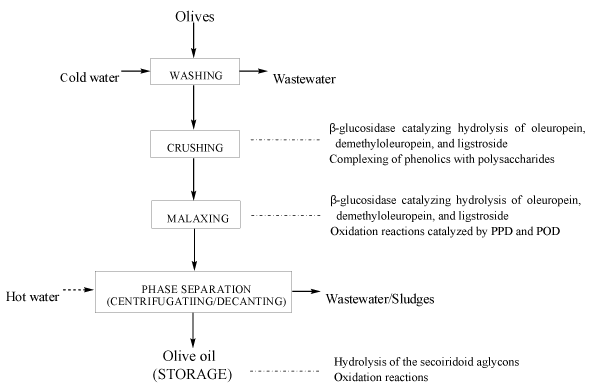
Secoiridoids are the predominant phenolic compounds in olive fruits which are exclusive to the plants of the Oleaceae family (Figure 1) [14]. Secoiridoids are characterised by the presence in their molecules of elenolic acid (EA, Figure 1 a) or its derivatives linked to 3, 4-dihydroxyphenylethanol (3,4-DHPEA/hydroxytyrosol, Figure 1 b ) or 4-hydroxyphenylethanol (4-HPEA/tyrosol, Figure 1 c ). Oleuropein (Figure 1 d), the 3,4-DHPEA ester of β-glucosylated elenolic acid, is an important secoiridoid mainly responsible for the bitterness of olive fruits [21]. Other major olive phenolics include demethyloleuropein (Figure 1 e), oleuropein aglycone (3,4-DHPEA-EA, Figure 1 f), ligstroside (Figure 1 g), ligstroside aglycon (4-HPEA-EA, Figure 1 h), the dialdehydic form of decarboxymethyl elenolic acid linked to 3,4-DHPEA (3,4-DHPEA-EDA/oleacein, Figure 1 i), and the related 4-HPEA-EDA (Figure 1 j) and nüzhenide (Figure 1 k) [22-24]. The most abundant secoiridoids in intact olives are oleuropein, demethyloleuropein, ligstroside, nüzhenide [25]. Oleuropein and demethyloleuropein are found mainly in the pericarp, whereas nüzhenide is found exclusively in the seed [25]. While, oleuropein is always present in the drupes of all the olive cultivars, demethyloleuropein and verbascoside can be cultivar-dependent [26].
Phenolic compounds are transferred to oil during processing and contribute to the sensory characteristics (bitter, astringent and pungent taste), stability and health benefits of olive oil. VOO contain higher levels of phenolics than refined olive oil due to losses occurring during oil refining [27]. The derivatives of the major secoiridoid constituent of olive fruit, oleuropein, form the majority of the phenolic fraction in the oil: 3,4-DHPEA, 3,4-DHPEA-EDA and 3,4-DHPEA-EA [2,29,30]. 4-HPEA, 3,4-DHPEA and 3,4-DHPEA-EA have been reported to constitute approximately 90% of VOO phenolics [27,31]. Notably, oleuropein and demethyloleuropein are absent in VOO, and there are two plausible explanations for this: (a) hydrolysis of the corresponding glucosides induced by endogenous β-glucosidases during the crushing and malaxation processes [32]; (b) the low solubility of these glucosidic substances in the oil phase [26].
Phenolic acids, such as caffeic, vanillic, syringic, 4-coumaric, 2-coumaric, protocatechuic, sinapic and 4-hydroxybenzoic acids, and flavonoids, such as luteolin, apigenin and taxifolin, have been also detected in VOO [29,33]. Luteolin and apigenin are the two main flavonoids present in VOO, which originate from their corresponding glucosides present in the olive fruit [34]. A new class of phenolic compounds 3,4-DHPEA-isochromans has been reported to occur in VOO [35], but it is still unclear whether they are natural compounds present in olive fruits or products formed during the technological "kneader" process or storage of oil.
Olive cultivar: There can be qualitative and quantitative differences in the phenolic composition of olive fruit depending upon the cultivar and where they are grown (country / region) [36]. The concentration of oleuropein has been observed to vary with the type of olive cultivar [37-39]. The Greek and Italian cultivars contain greater amounts of oleuropein in comparison with the Spanish and Portuguese cultivars [40], and slow-ripening cultivars contain more oleuropein and its aglycone than fast-ripening cultivars [23]. In two cultivars grown in the south of Tunisia, oleuropein was abundant in the Gemri-Dhokar cultivar (61.04 mg/100 g of fresh olive) but occurred at relatively much lower concentrations in the Dhokar cultivar (0.25 mg/100 g of fresh olive) according to HPLC analysis [41]. This explained the distinctive sweet taste of the Dhokar cultivar. A similar result was reported by Jemaiet al. where Dhokr cultivar contained much lower concentrations of phenolics compared to Chemlali cultivar, being 0.16 g Gallic Acid (GA) equivalents/kg and 3.75g GA equivalents/kg of fresh olive respectively at harvest [42]. Koroneiki cultivar, an indigenous Greek olive variety, is thought to be resistant to verticillium wilt by involvement of phenolic compounds in the defense mechanism compared with the susceptible Amfissis cultivar [43].
3,4-DHPEA and oleuropein were the most abundant phenolic compounds identified in 18 different olive cultivars from north and central Portugal, where rutin and luteolin 7-O-glucoside were the two main flavonoids [44]. 3,4-DHPEA has been reported as the major phenolic compound in the olive cultivars Ayvalık, Domat and Gemlik where its concentration generally exceeds that of oleuropein [37]. Demethyloleuropein only occurs in the cultivars of Coratina and Leccino and not other Italian cultivars. Indeed, this compound has been recommended as a potential marker for these particular cultivars [45]. Large differences in the concentration of verbascoside have been observed in the olive cultivars of Picual (Spain), Frantoio (Italy), Salonenque (France) and Galega (Portugal), raising the prospect of using this compound as a marker for differentiating these cultivars harvested at the same maturity [36]. Similarly, the ratio of anthocyanins to anthocyanins derivatives has been proposed as a parameter to differentiate five Italian cultivars, namely Frantoio, Rossellino, Ciliegino, Cuoricino, and Grossolana [22]. A new phenolic compound 3-O-galloyl quinic acid butyl ester has been reported from the fruit pulp of a Chinese olive variety, but it is still uncertain whether this compound is unique to this variety [46].
The maturation stages of olive fruits represented by green, spotted, purple and black fruit colours correspond to the concentration of anthocyanins [47]. Differences in the phenolic composition between mature and immature fruits have been attributed to chemical and enzymatic reactions occurring during the ripening process.
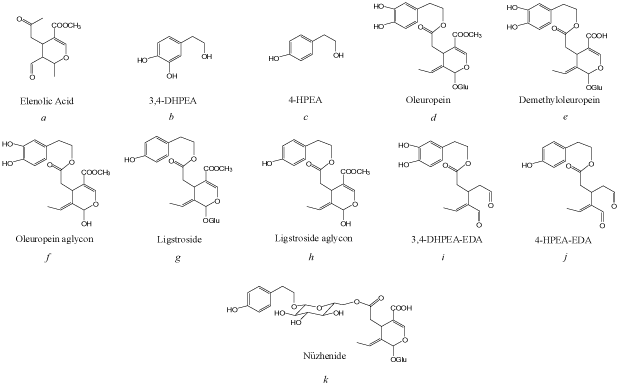
Oleuropein and ligstroside which are the major secoiroidoids constituents of unripe olive fruit have been reported to decrease during ripening and is almost undetectable in dark olive fruits [48,49]. This is accompanied by increases in the concentrations of 3,4-DHPEA, 4-HPEA, and verbascoside [50], as well as that of the nonphenolic compound elenolic acid glucoside [51]. The degradation of oleuropein and ligstroside as shown in Figure 2 could be due to the increased activity of hydrolytic enzymes and esterase during ripening [42,50], producing oleuropein aglycone and glucose by β-glucosidase enzyme hydrolysis followed by degradation into elenolic acid and 3,4-DHPEA by esterase [42]. Since oleuropein and ligstroside degrade into aglycones during ripening, it has been suggested that elenolic acid glucoside and 3,4-DHPEA might serve as potential indicators of olive maturity [45]. However, in Chétoui cultivar, oleuropein and 3,4-DHPEA in olive fruit exhibited the same trends during maturation, and the concentration of oleuropein was not inversely correlated with the concentration of 3,4-DHPEA [52]. Polymerization of oleuropein might explain the decrease in oleuropein concentration with the formation of phenolic oligomers [21], as evidenced by the detection of oleuropein trimers in Chétoui olive extracts at harvest [51]. Similarly, changes in the concentration of phenolic acids, such as trans-cinnamic acid, sinapic acid, 4-coumaric acid and syringic acid, exhibit cultivar based differences during ripening [53].
An apparent decrease in the concentrations of sugars and phenolics during maturation of olive fruit has been reported [39], which might be attributed to a reaction between the two [54]. Flavonoid glucosides, mainly luteolin 7-O-glucoside, initially increased during green maturation and later declined, accompanied by an increase in the concentration of the flavonoid aglycones at the last stage of ripening [55]. L-Phenylalanine Ammonia Lyase (PAL) is the key enzyme in phenolic biosynthesis. A decrease in enzymatic activity of PAL is a plausible explanation for the reduction in total phenolic content of olive drupes during ripening [37,39]. In contrast to the above observations, Bouazizet al. reported that the olive cultivar Chemlali presented the highest concentration of phenolics at the last stage of maturation [55].
In addition to the cultivars and maturity, irrigation also affects the composition of phenolic compounds in olive fruits [56]. Irrigation had negligible effects on free acidity, peroxide value, and fatty acid composition of VOO, but strongly influenced its phenolic concentration [57]. It is generally accepted that phenolic substances are more abundant in drought-stressed olive crops than in irrigated crops, which might be due to draught-related variation in the enzymatic activities involved in the formation of phenolic substances. It has been reported that the activity of the enzyme PAL in olive fruit decreased with increased irrigation [58,59].
Machado et al. observed that the phenolic content, PAL and antioxidant activity of olive fruits of the Portuguese cultivar Cobrançosa were significantly affected by the extent of water treatments applied, giving 61.3 to 15.7 g GA equivalents/kg under rain fed conditions (no irrigation), 24.5 to 11.2 g GA equivalents/kg under inadequate irrigation and 14.7 to 10.2 g GA equivalents/kg under good irrigation [56]. Providing irrigation from the second half of the fruit growing cycle (i.e. after pit hardening) can delay the ripening process leading to changes in the primary and secondary metabolisms, resulting in marked changes in the phenolic composition at harvest [60]. Thus, irrigation exerts a negative effect on the quality of VOO by way of diminished phenolic concentrations.
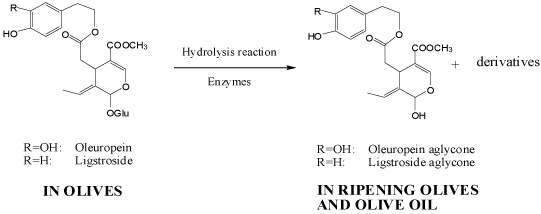
The extraction process for VOO production comprises four main stages: crushing, malaxation, separation and clarification (Figure 3). During oil extraction, phenolic compounds in olive fruit are transferred from crushed fruit (paste) to olive oil and by-products (pomace and wastewater). The amount of phenolic compounds transferred from fruit to oil is influenced by the solubility of these compounds in the oil phase and the presence of surfactants and temperature [18]. In addition, reactions such as breakdown of glycosides and oligosaccharides by glucosidases, oxidation by phenol oxidases, and polymerization of free phenols result in modification of olive phenolics [61,62].
Crushing is performed to break down cell walls and release oil droplets within the cells, which can also trigger several enzymatic reactions resulting in the conversion of phenolic compounds into various products as well as generation of volatile flavor compounds. Thus, depending on the crushing conditions, the concentration of minor components intimately related to the taste, aroma and stability of VOO are modified. Generally, the stronger the crushing conditions, the higher the concentration of phenolic compounds in both olive paste and oil. This could be attributed to greater disintegration of tissue as well as activation of enzymes such as β-glucosidase [62]. Consequent enzyme activity results in the generation of aglycones such as 3,4-DHPEA-EDA and 4-HPEA-EDA. 3,4-DHPEA-EDA could be obtained from the hydrolysis of oleuropein, demethyloleuropein, and ligstroside [19]. A large fall in the oleuropein content of olive paste has been observed [26,63], suggesting extensive degradation/ conversion during the crushing operation. In addition to enzymatic reactions, phenolic compounds may also be complex with several polysaccharides impairing their access to the oil phase during crushing and malaxation [64].
Malaxation induces coalescence of small oil droplets into larger droplets and subsequent formation of a continuous oil phase, and promotes release of phenolic compounds into oil phase. And the activation of endogenous β-glucosidases during crushing can catalyze the hydrolysis of oleuropein, demethyloleuropein and ligstroside leading to the generation of their aglyconic forms [65]. Both malaxation temperature and time could influence the phenolic composition of VOO [17,66,68]. Interactions between polysaccharides and phenolic compounds present in the olive pastes may also be involved in the loss of phenols during malaxation process [26].
In experiments performed under laboratory conditions, the concentration of hydrophilic phenols (simple phenols, secoiridoids and lignans) in oil increased with the malaxation temperature attaining a maximum at 27 °C [69]. The concentration of the simple phenols increased linearly up to 36°C which was the maximum malaxation temperature examined, and the concentration of lignans was virtually unaffected. Stefanoudaki et al. also found a large increase in the phenolic concentration of VOO when the malaxation temperature was raised between 15 and 42 °C for two popular Italian (cv. Coratina) and Cretan (cv. Koroneiki) varieties [68]. On the other hand, other studies have shown falls in the phenolic content when the malaxation temperature was increased beyond 27 °C [17,69]. This fall in the phenolic concentration at higher malaxation temperatures has been attributed to oxidative degradation of secoiridoids resulting from enhanced activity of native oxidoreductases, e.g. Polyphenoloxidases (PPO) and Peroxidases (POD) and Lipoxygenases (LOX) [70]. Hydrolysis of oleuropein and demethlyoleuropein to their corresponding aglycones by β-glucosidase has also been suggested as a reason for the fall in phenolic concentration at higher malaxation temperatures [17].
The opinion that malaxation temperatures higher than 27 °C lowers the phenolic concentration and thereby the quality of VOO has been brought in to question by more recent studies [66,71]. The terms 'first cold pressing' and 'cold extraction' signifying better quality VOO can only be used for oils extracted below 27 °C. However, VOO produced in an industrial plant using a malaxation temperature of 35 °C has been shown to contain more phenolic compounds, greater storage stability and better sensory properties than oil produced at 25 °C [66]. Taticchi et al. reported that whilst POD was essentially stable up to 40 °C, PPO was thermally labile with low thermal stability even at 40 °C [71]. Other contributory factors currently not well understood are the influence of malaxation temperature on the release of phenolics from cell wall polysaccharides by endogenous hemicellulases [64], and polygalcturonases and the solubilisation of the releasaed phenolics in VOO [71].
Malaxation time also influences the phenolic content of VOO. In general, the phenolic concentration decreases as the malaxation time is increased. This is probably due to oxidative degradation of the phenolics catalysed by PPO, POD and LOX which increases with the exposure time of olive paste to air. Ranalli et al. recommended that the malaxtion time should not exceed 45 min in order to preserve the phenolics [72]. Chemlali and Chetoui VOOs presented the highest total phenolic concentrations equal to 230.58 and 828.72 mg GA equivalents/kg at malaxation time of 30 min while the lowest concentrations at 60 min. In addition, the composition of individual phenolic compounds was differentially influenced by malaxation time [73]. Artajo et al. reported that malaxation time had an important effect on the alcohols and secoiridoids: the concentration of 3,4-DHPEA and 4-HPEA decreased and their hydrophilic character improved through their presence in the wet pomace and wastewater; the concentrations of demethyloleuropein and oleuropein decreased significantly after 45 min of malaxation, which indicated the beginning of the degradation process of secoiridoid compounds or their transformation into secoiridoid derivatives, mainly 3,4-DHPEA-EDA [74]. Jiménez et al. also reported that the malaxation time of olive paste was negatively correlated with the concentrations of secoiridoid as well as 3,4-DHPEA and 4-HPEA, but had little effect on the concentrations of phenolic acids, flavonoids and lignan [71]. Nevertheless, the effects of malaxation time on the phenolic content of VOO are minor compared to the effects of malaxation temperature [17,63,68].
Commercial VOO production is performed using either a classical mill (batch process) or a centrifugal extractor (continuous process). The classical mill, used for centuries, has been almost replaced by centrifugal extractors because of the productivity gains it offers [68]. The centrifugal process can be either a two-phase or three-phase separation system. The two-phase system, introduced relatively more recently, requires a minimal moisture value on the olive paste (50%) to facilitate the separation process [76]. This system yields higher levels of phenolics, especially 3,4-DHPEA and 4-HPEA, and more stable oil compared to three-phase systems [77,78]. Klen and Vodopivec reported that the phase separation method only impacts on the quantitative recovery of phenolics and had no obvious qualitative effect on the phenolic composition [79]. In contrast to the three-phase systems, the two-phase extractors do not produce waste water and produce VOO containing higher concentrations of phenolics as well as flavour compounds [80].
Compared to other edible oils, VOO is reputed to be stable to oil processing and storage conditions [32]. This has been attributed in part to the antioxidant effects of the phenolic compounds present in VOO [20]. It has been shown that the concentrations of total phenolics and the oleosidic forms of 3,4-DHPEA correlated (r=0.97) with the oxidative stability of VOO [81]. Both hydrolysis and nonenzymatic oxidation of phenolics can take place during storage, leading to a decrease in VOO secoiridoid content and an increase in simple phenolics (such as 3,4-DHPEA and 4-HPEA), elenolic acid, oxidised forms of elenolic acid and oxidised forms of secoiridoids, whereas lignans, 1-acetoxypinoresinol and pinoresinol were relatively stable [82-84]. Brenes et al. observed that hydrolysis of secoiridoid aglycons was the main change to the phenolic constituents in the VOOs of Arbequina, Hojiblanca, and Picual varieties during storage in the absence of light, resulting in an increase the contents of 3,4-DHPEA and 4-HPEA in these oils [83]. Okogeri and Tasioula-Margari studied the trends in the concentrations of phenolics during storage of VOO, and found that the concentrations of total phenolics decreased by 57−63% after 6 months under diffused light compared to39−45% in the dark [85]. A decrease in the VOO phenolic concentration by as much as 81% after storage for 9 months has been reported [86].
A positive correlation has been found between the storage age of VOO and the ratio of 4-HPEA to total phenolics [87], and the ratio of 4-HPEA to total phenolics has been proposed as an indicator for distinguishing freshly made oils from oils stored for substantial periods (<4% for 6 month storage, >42% for longer than 18 month storage). 3,4-DHPEA-EDA degradation products are also suggested as molecular markers of the early auto-oxidation state of VOO, which could be an early evaluation index of VOO shelf life [88].
Consumption of olives and olive oil has been recognized as an important way to increase the dietary intake of phenolic compounds and derive the health benefits associated with these bioactive compounds. Olive cultivar, irrigation and the degree of ripening have major qualitative and quantitative effects on the phenolic constituents in olive fruit. Phenolic compounds are changed both qualitatively and quantitatively during the transfer from olive fruit to olive oil, resulting in quite different phenolic composition of VOO compared to that of olive fruit. Since only about 2% of phenolics in olive fruit are transferred to VOO and the rest ends up in the pomace or wastewater depending on the extraction system used, the phenolics present in pomace and wastewater could serve as potential sources of valuable phenolic compounds for edible use.
The main classes of phenolic compounds of olive fruit.
Class |
Phenolic compounds |
Reference |
Phenolic acids |
Chlorogenic acid, Caffeic acid, 4-Hydroxybenzoic acid, Protocatechuic acid, Vanilic acid, Syringic acid, 4-Coumaric acid, 2-Coumaric acid, Ferulic acid, Sinapicacid, Benzoic acid, Cinnamic acid, Gallic acid, Caffeoylquinic acid, 3,4-Dihydroxyphenyl-propionic acid |
[14,21,61] |
Phenolic alcohols |
3,4-Dihydroxyphenylethanol (3,4-DHPEA), 4-Hydroxyphenylethanol (4-HPEA), Homovanillyl alcohol (the metabolite of 3,4-DHPEA) |
[22,45,55] |
Flavonoids or the glucosides |
Quercetin, Luteolin, Apigenin, Chrysoeriol, Rutin, Quercetin-3-O-rutinoside, Luteolin-7-O-glucoside, Luteolin-5-O-glucoside, Apigenin-7-O-glucoside, Apigenin-7-O-rutinoside, Apigenin-6,8-glucoside, Chrysoeriol-7-O-glucoside, Luteolin-4-glucoside |
[21,55,61] |
Secoiridoids or the hydrolytic derivatives |
Oleuropein, Ligstroside, Nüzhenide, Elenoic acid, Demethyloleuropein, Oleuropein aglycone (3,4-DHPEA-EA), the dialdehydic form of decarboxymethyl elenolic acid linked to 3,4-DHPEA (3,4-DHPEA-EDA), Ligstroside aglycone (4-HPEA-EA), the dialdehydic form of decarboxymethyl elenolic acid linked to4-HPEA (4-HPEA-EDA) |
[14,22,24] |
Anthocyanins |
Cyanidin-3-O-glucoside, Cyanidin-3-O-rutinoside, Cyanidin-3-caffeyglucoside, Cyanidin-3-caffeylaitinoside, Delphinidin 3-rhamosylglucoside-7-xyloside |
[14,22,24] |
Hydroxycinnamic acid derivatives |
Verbascoside |
[70] |
Molecular structures of the major phenolic compounds in olive fruit.
Degradation of oleuropein and ligstroside into aglycones during ripening. (adapted from Vissers et al., 2002).
Unit operations in olive oil extraction and their impact on phenolic compounds.